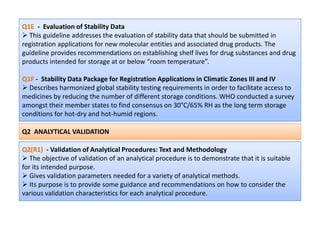
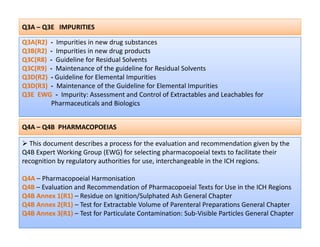
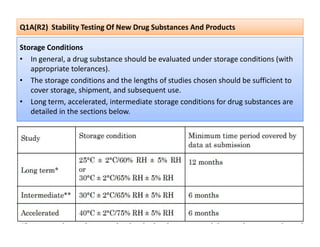
The document discusses guidelines from the International Conference on Harmonization (ICH) related to quality, safety, and efficacy of pharmaceuticals. It provides an overview of the ICH process and objectives to harmonize technical requirements for drugs internationally. Key points include that ICH aims to reduce duplicative testing, make approval processes more efficient, and ultimately benefit consumers and patients. Guidelines cover topics such as stability testing, impurities, good manufacturing practices, clinical trial standards, and more. The document outlines the six ICH member regions and five step process for establishing new harmonized guidelines.