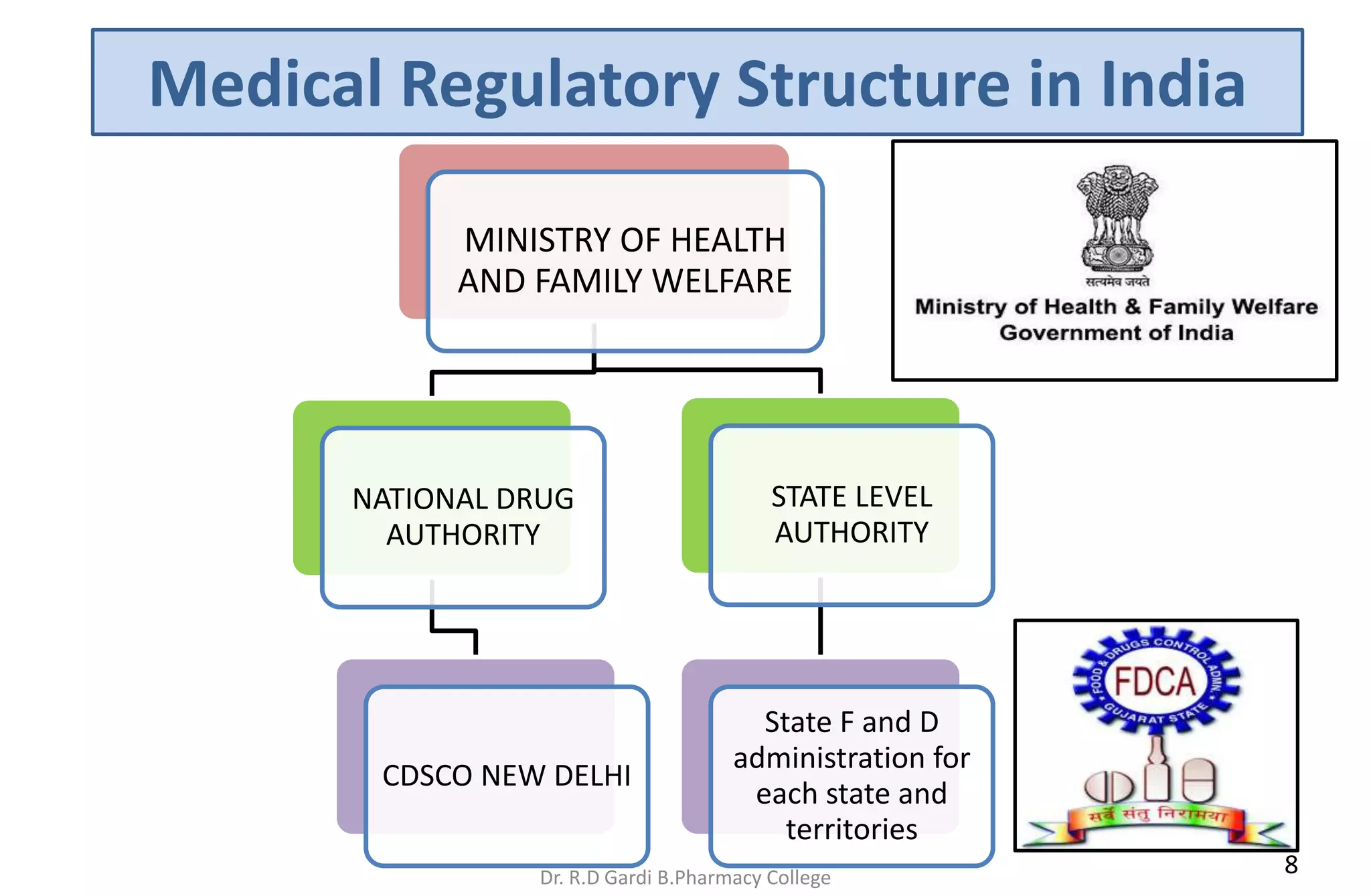
This document discusses approved regulatory bodies and agencies that regulate drugs. It provides information on regulatory authorities in countries like the US, EU, India, Japan, and their functions. The key regulatory bodies mentioned are the US FDA, EU, India's CDSCO under the Ministry of Health and Family Welfare. The functions of regulatory bodies include drug evaluation, monitoring safety and efficacy, licensing, and adverse reaction monitoring. They aim to ensure drug safety, efficacy, and quality. Harmonization of regulatory standards globally and strengthening authorities are needed to regulate the pharmaceutical industry effectively.