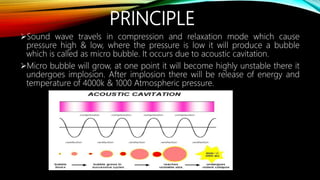
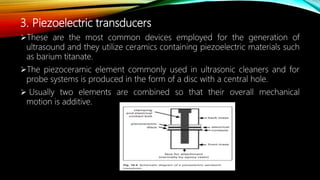
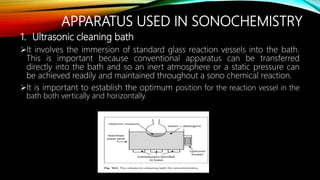
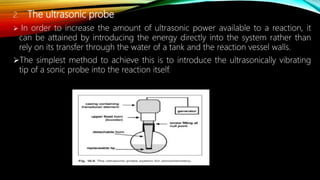
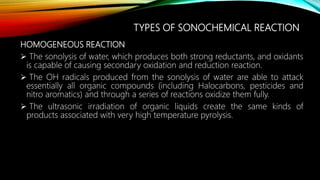
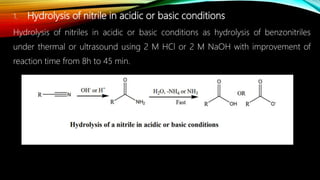
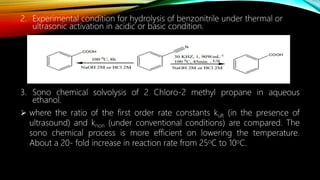
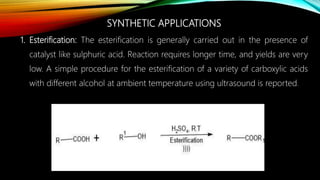
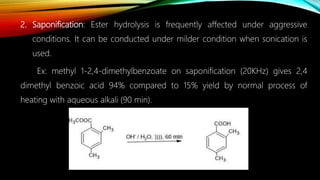
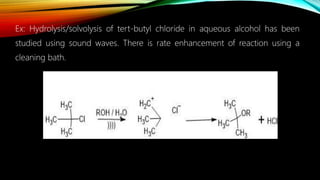
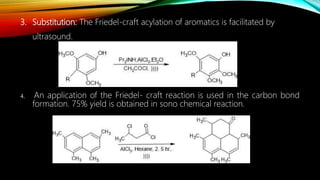
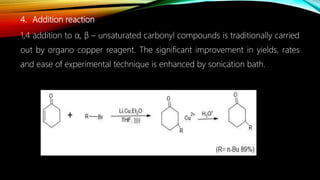
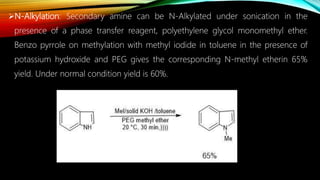
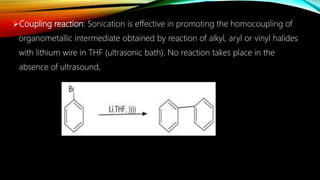
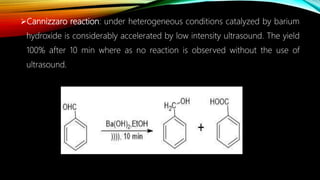
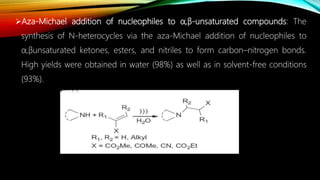
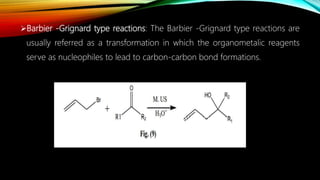
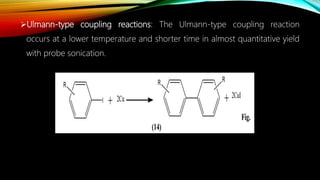
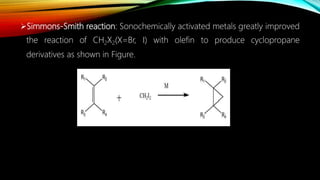
Ultrasound assisted reactions can enhance chemical synthesis through cavitation effects. Piezoelectric transducers are commonly used to generate ultrasound from 20 kHz to 2 MHz. Cavitation produces localized hot spots exceeding 4000K that can drive homogeneous and heterogeneous reactions. Homogeneous reactions involve single-phase systems and produce radicals from water sonolysis. Heterogeneous reactions involve multi-phase systems and benefit from improved mixing and mass transfer. Many reactions like esterification, hydrolysis, substitution, and addition have been achieved with higher yields and faster reaction times using ultrasound.