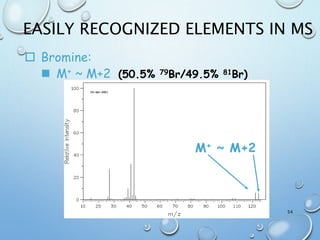
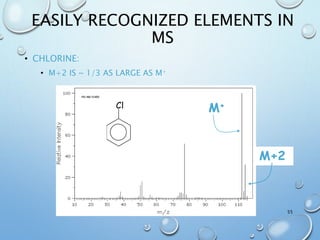
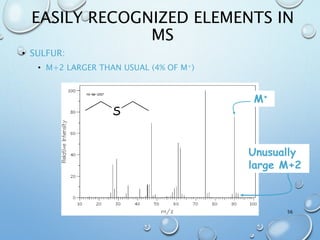
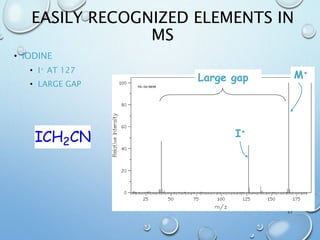
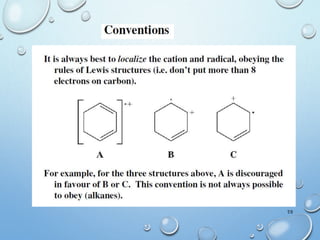
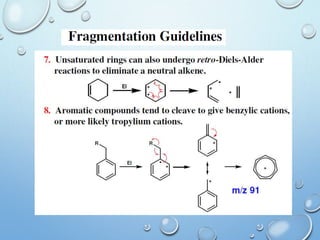
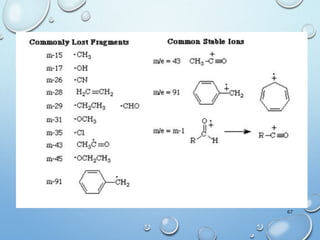
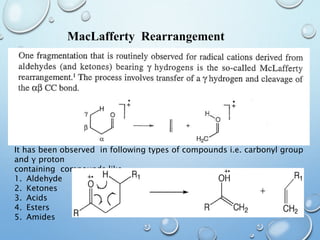
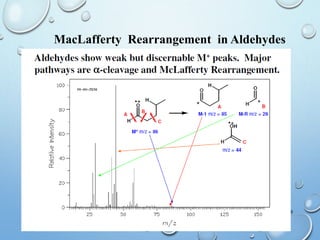
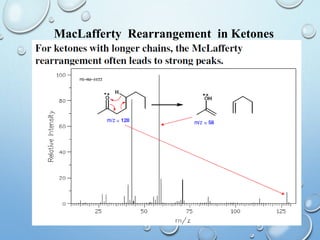
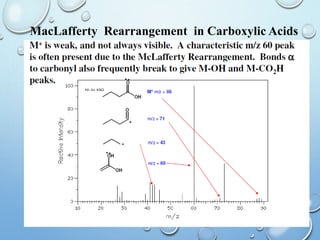
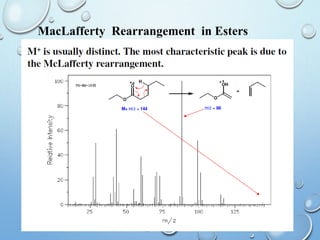
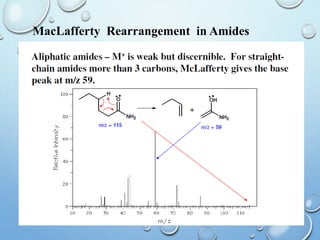
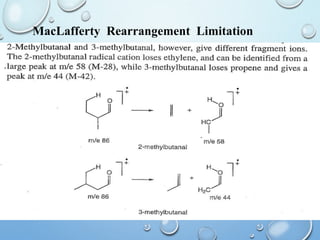
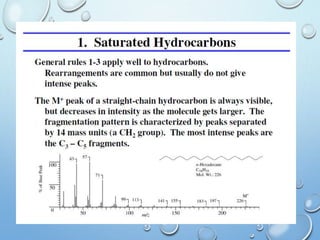
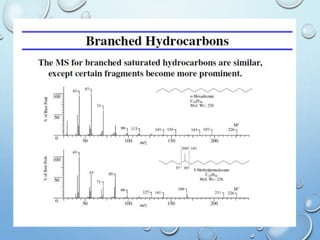
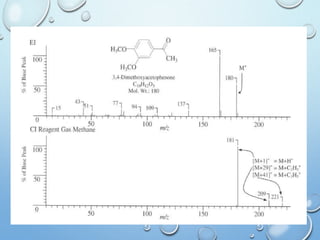
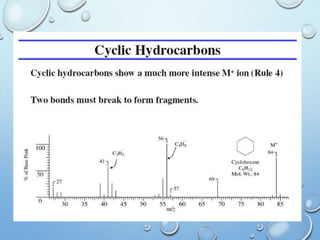
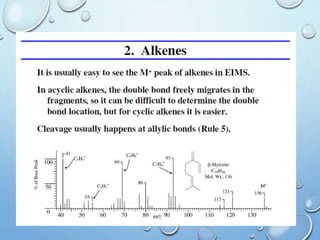
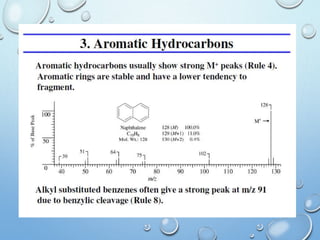
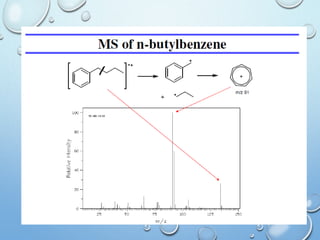
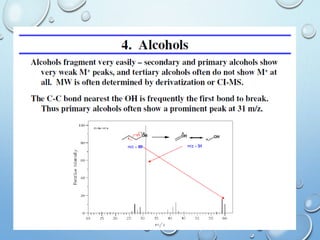
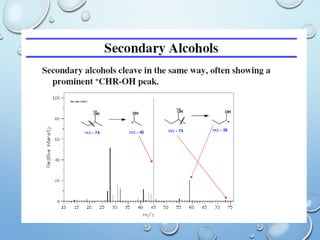
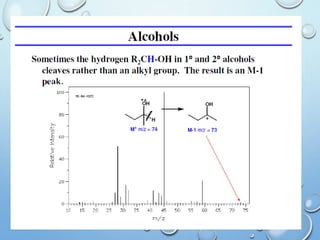
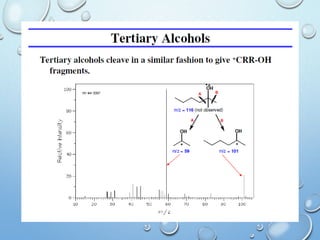
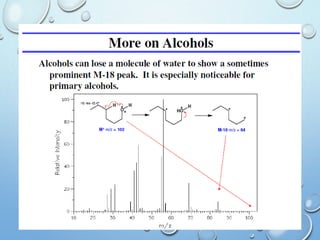
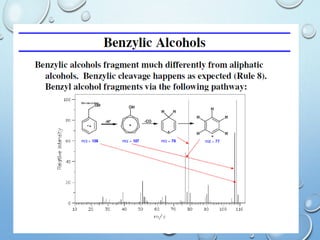
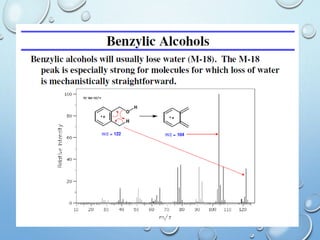
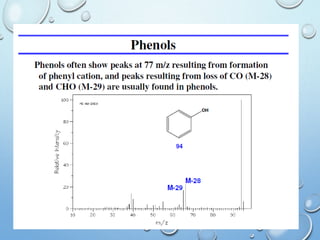
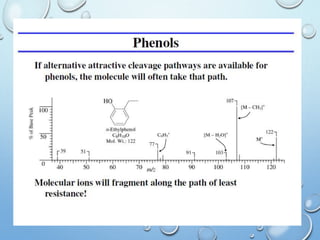
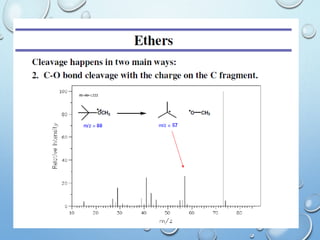
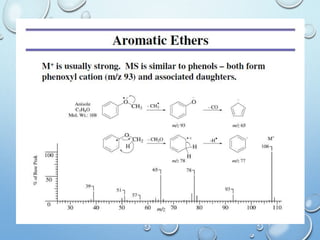
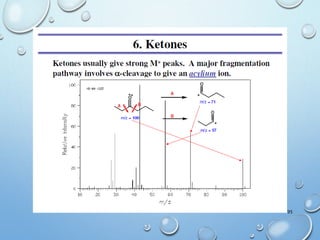
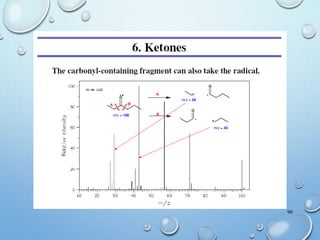
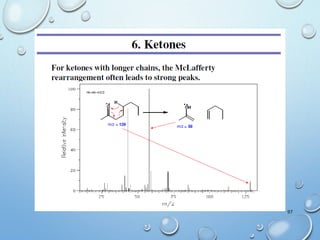
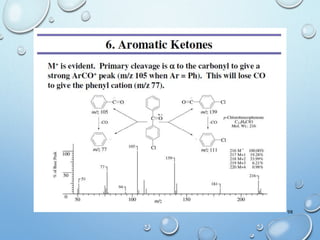
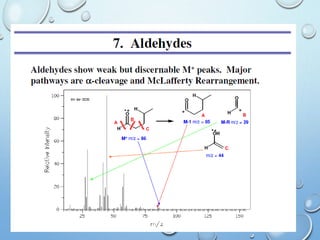
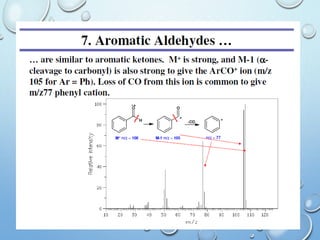
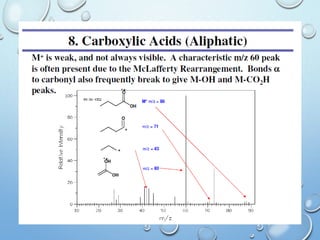
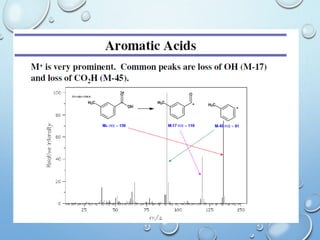
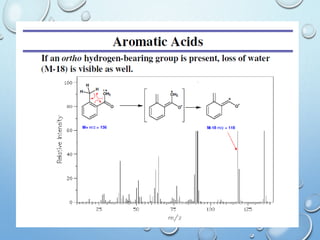
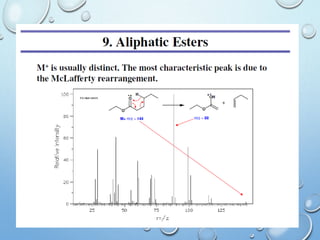
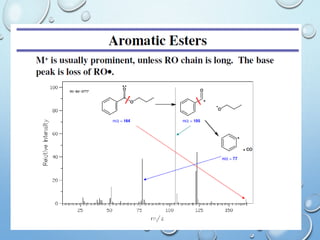
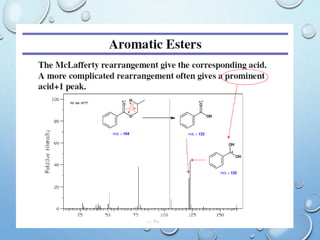
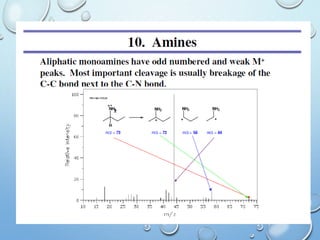
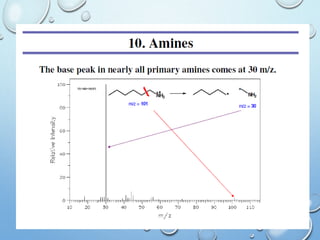
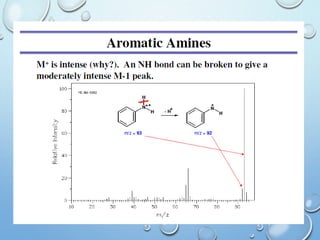
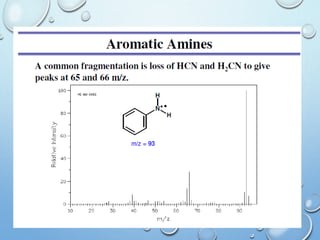
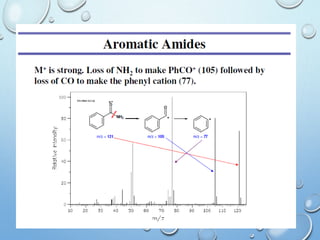
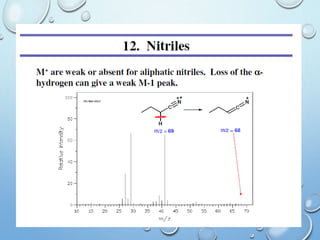
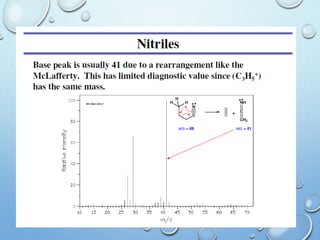
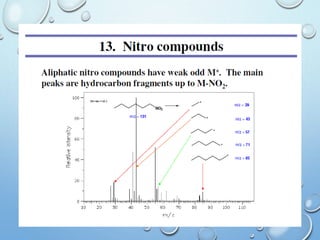
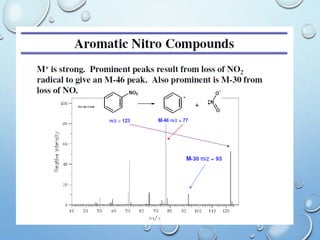
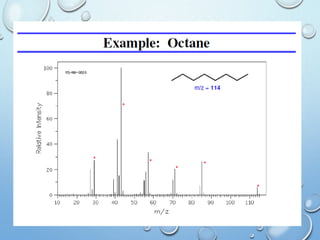
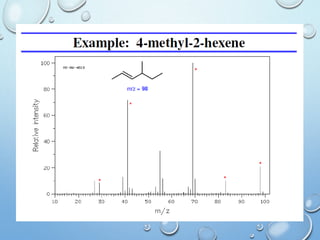
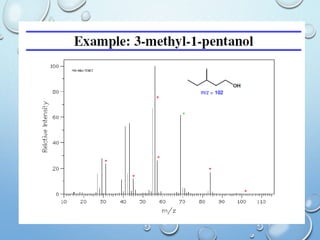
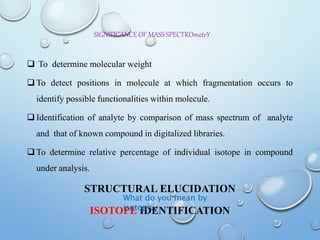
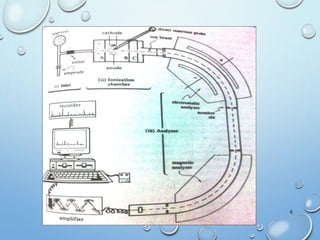
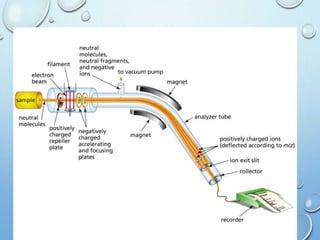
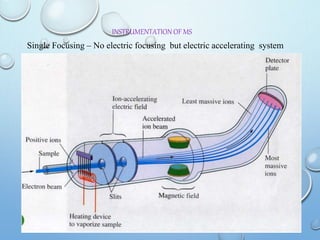
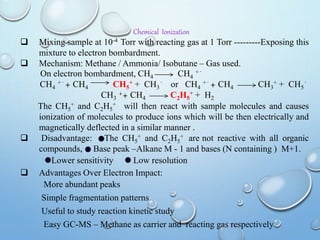
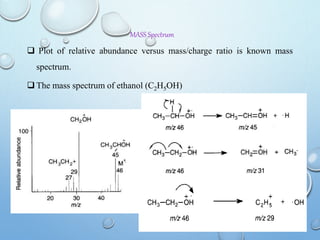
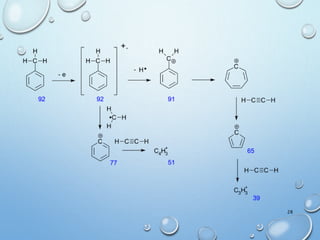
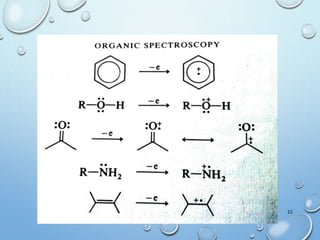
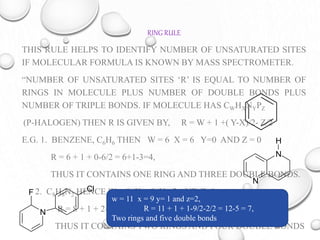
The document discusses the principles and significance of mass spectrometry, detailing the ionization methods and mechanisms of fragment ion generation. It explains various ionization techniques, such as electron impact and chemical ionization, alongside their advantages and limitations. Additionally, it outlines the role of mass spectra in determining molecular weights and analyzing the composition of substances.

![principlE OF MASS SPECTROmetrY
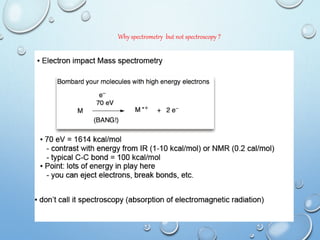
Organic molecules (M) on electron bombardment are converted to
highly energetic positively charged ion [M + ֹ ] or negatively charge ion
[M - ֹ ] (Molecular / Parent ion)
M - e M + ֹ or M + e M - ֹ
Positive ion MS is more probable than negative ion by 102 factor.
The loss of electron from molecule gives molecular or parent ion.
Molecular ion will then degraded in to its constituent ions known as
fragment ions or daughter ions at possible breaking points within
molecule.
What is ionization potential ?](https://image.slidesharecdn.com/massspectroscopy-191129144946/85/Mass-spectroscopy-3-320.jpg)

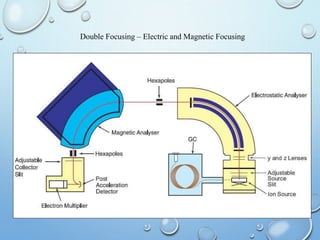


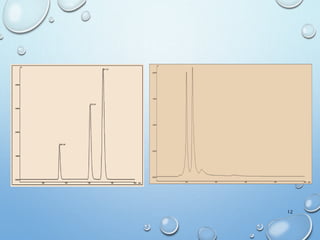


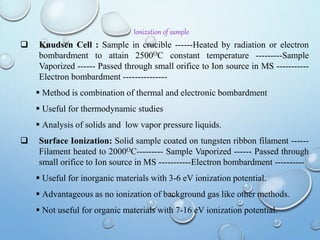
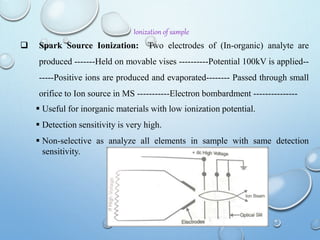









![MOLECULAR IONS/ PARENT PEAK
AS LIKE UV SPECTROSCOPY, WE ARE NOT CONCERNED WITH
ELECTRONIC EXCITATIONS AS ENERGY OF ELECTRON IMPACT IS
70 EV WHICH LOSSES SPECIFICITY OF ATTACK ON MOLECULE I.E.
IT IS UNABLE TO JUDGE ORBITAL OF ELECTRON REMOVAL IN
MOLECULE.
HENCE WHEN ELECTRONS IS REMOVED FROM HOMO, IT SHOULD
BE CONSIDERED THAT IT IS REMOVED FROM MOLECULE AS
WHOLE.
HENCE TO REPRESENT MOLECULAR ION, EITHER OR BOTH OF
FOLLOWING METHODS, PARTIAL /COMPLETE SQUARE BRACKET,
[C2H5] + ֹ / C2H5˥ + ֹ OR
FRAGMENTATION OF MOLECULAR ION IS NOT SIMPLE PROCESS. IT
NEEDS VARIOUS CONSIDERATIONS LIKE REACTIVITY OF](https://image.slidesharecdn.com/massspectroscopy-191129144946/85/Mass-spectroscopy-31-320.jpg)







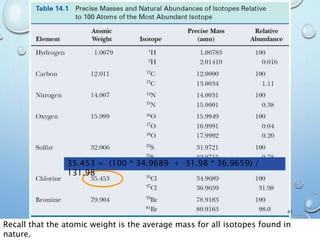
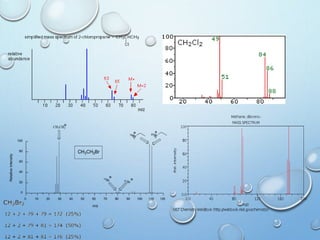
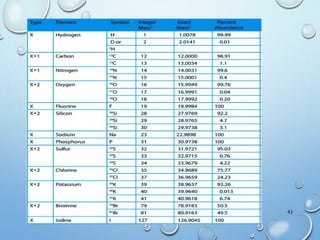
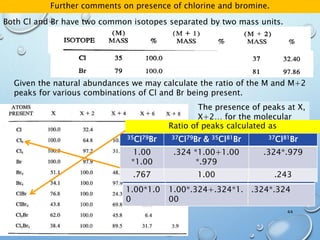

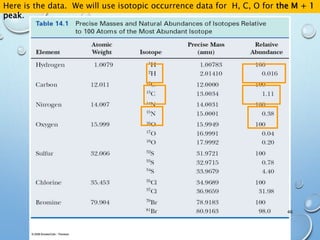

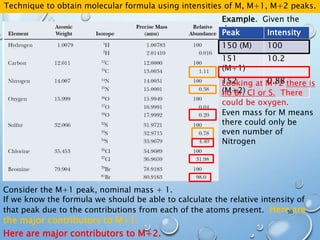
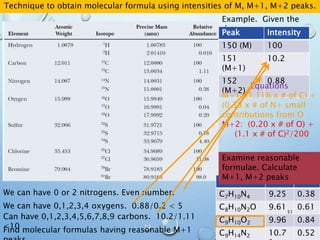
![M+2 PEAK, CONTRIBUTIONS FROM ONE ATOM
AND TWO ATOMS.
49
Recap:
The M+1 peak has contributions from one atom being a heavier isotope by 1.
(M+1)/M = ca. 1.1% * no. of C atoms + 0.36% * no. of N atoms
The M+2 peak can have contributions from two sources
•One atom being a heavier isotope by 2. Mainly O (excluding S, Cl and Br)
•Two atoms being heavier by 1 each. Mainly C atoms.
(M+2)/M = ca. (0.20% * no. of O atoms) + (1.1 * no. of C atoms)2/200%
Example 1: C5H5N
[(A + 1)+]/[A+] = 5 x 1.1% + 1 x 0.36%
= 5.9%
[(A + 2)+]/[A+] = 5.52/200 % = 0.15%
Example 2: C7H5O
[(A + 1)+]/[A+] = 7 x 1.1% = 7.7%
[(A + 2)+]/[A+] = 7.72/200 % + 0.20% =
0.50%](https://image.slidesharecdn.com/massspectroscopy-191129144946/85/Mass-spectroscopy-48-320.jpg)