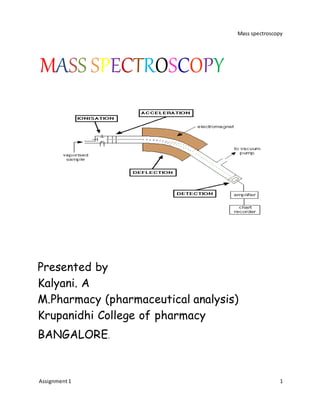
Mass spectrometry is a technique used to identify unknown compounds and determine molecular structure. It works by ionizing sample molecules and measuring their mass-to-charge ratios. The document discusses various components of a mass spectrometer including the inlet system, ionization sources, and mass analyzer. Common ionization methods like electrospray ionization (ESI), matrix-assisted laser desorption/ionization (MALDI), fast atom bombardment (FAB), and electron impact (EI) are described in detail, outlining their principles, advantages, and applications. The mass spectrum provides information about molecular weight and structure of compounds.






![Mass spectroscopy
Assignment1 8
c. Probing molecular dynamics
d. Monitoring chemical reactions and studying reactive intermediates.
e. Chemical imaging.
f. Identification and quantification of hemoglobin variants.
g. Screening for inborn errors of metabolism.
MALDI (Matrix AssistedLaser DesorptionIonization)
MALDI is also based on “soft ionization” methods where ion formation does not lead to
a significant loss of sample integrity.
Consequently, the high throughput and speed associated with complete automation has
made MALDI-TOF mass spectrometer an obvious choice for proteomics work on large-
scale.
Advantages
a. Gentle Ionization technique
b. High molecular weight analyte can be ionized
c. Molecule need not be volatile
d. Wide array of matrices
e. Produces singly charged ions thus interpretation becomes easy.
f. Prior separation by chromatography is not required.
Disadvantages
a. MALDI matrix cluster ions obscure low m/z species (<600)
b. Analyte must have very low vapor pressure
c. Pulsed nature of source limits compatibility with many mass analyzers
d. Coupling MALDI with chromatography can be difficult
e. Analytes that absorb the laser can be problematic.
MALDI – Principle
The sample for analysis by MALDI MS is prepared by mixing or coating with solution of an
energy-absorbent, organic compound called matrix. When the matrix crystallizes on drying, the
sample entrapped within the matrix also co-crystallizes. The sample within the matrix is ionized
in an automated mode with a laser beam. Desorption and ionization with the laser beam
generates singly protonated ions from analytes in the sample.
Matrix and Sample Preparation
The matrix consists of crystallized molecules, of which the three most commonly used
are - 3, 5-dimethoxy-4-hydroxycinnamic acid (sinapinic acid), α-cyano-4- hydroxy
cinnamic acid (CHCA) and 2, 5-dihydroxybenzoic acid (DHB).
A solution of one of these molecules is made, often in a mixture of highly purified water
and an organic solvent such as acetonitrile (ACN) or ethanol.
A counter ion source such as Trifluoroacetic acid (TFA) is usually added to generate the
[M+H] ions.
A good example of a matrix solution would be 20 mg/ml sinapinic acid in ACN: water:
TFA (50:50:0.1).
The matrix performs two important functions:
1. It absorbs photon energy from the laser beam and transfers it into excitation energy of the
solid system,](https://image.slidesharecdn.com/massassingment-190322130213/85/Mass-assingment-8-320.jpg)



![Mass spectroscopy
Assignment1
12
Plasma Desorption
• Plasma desorption produces molecular ions from the samples coated on a thin foil when a
highly energetic fission fragments from the Californium-252 “blast through” from the opposite
side of the foil.
• The fission of Californium-252 nucleus is highly exothermic and the energy released is carried
away by a wide range of fission fragments which are heavy atomic ion pairs.
• Ion pair fission fragments depart in opposite directions.
• Each fission of this radioactive nucleus gives rise to two fragments traveling in opposite
directions (because necessity of momentum conversation).
• A typical pair of fission fragments is 142Ba18+ and 106TC22+, with kinetic energies roughly
79 and 104 MeV respectively.
• When such a high energy fission fragments passes through the sample foil, extremely rapid
localized heating occurs, producing a temperature in the range of 10000K.
• Consequently, the molecules in this plasma zone are desorbed, with the production of both
positive and negative ions.
• These ions are then accelerated out of the source in to the analyzer system.
Atmospheric Pressure Chemical Ionization
(APCI)
APCI is an ionization method used in mass spectrometry (commonly LC-MS) which
utilizes gas-phase ion-molecule reactions at atmospheric pressure.
It is an ionization method that is similar to chemical ionization (commonly used in GC-
MS) where corona discharges on a solvent spray produce primary ions.
APCI is mainly used with polar and relatively non-polar compounds with a molecular
weight of less than 1500 Da, generally giving mono-charged ions.
Advantages
a. Multiple charging is typically not observed as the ionization process is more energetic than
ESI.
b. Electron transfer or proton loss, ([M-H]-) occurs in the negative mode.
c. Proton transfer (for protonation MH+ reactions) occurs in the positive mode
d. At atmospheric pressure analyte molecules collide with the reagent ions frequently and hence
ionization is very efficient.](https://image.slidesharecdn.com/massassingment-190322130213/85/Mass-assingment-12-320.jpg)
![Mass spectroscopy
Assignment1
13
Limitations
a. Very sensitive to contaminants such as alkali metals or basic compounds.
b. Relatively low ion currents.
c. Relatively complex hardware compared to other ion sources.
PRINCIPLE
In APCI, the sample is typically dissolved in a solvent and pumped through a capillary
inside an uncharged quartz tube. At the end of the capillary, but still within the tube, the
sample is converted into an aerosol and then vaporized with the help of nitrogen gas and
by heating to very high temperature (~350- 550 °C).
The gaseous solvent (S) and sample (M) are then ionized by a corona discharge, in
which a highly charged electrode creates an electric field strong enough to ionize nearby
molecules.
A potential of several kilovolts applied to the electrode typically remove an electron
from a neutral molecule, without depositing enough internal energy to cause
fragmentation.
The corona discharge may directly ionize an analyte molecule to form a radical cation
(M+●): M + e- → M+● + 2e-
S + e- → S+● + 2e-
Frequent collisions between the ions and molecules can transfer charge from an ion to
another neutral. Collision of an ionized solvent ion with an analyte molecule can create a
direct charge transfer to form a radical cation analyte ion:
S+● + M → M+● + S
Alternatively, collision of solvent ions with a neutral analyte molecule may result in
abstraction of a hydrogen atom from the molecule. The resulting ionized solvent can then
ionize the analyte via proton transfer:
S+● + S → [S+H]+ + S [-H]
[S+H]+ + M → [M+H]+ + S
The resulting analyte ions (M+● or [M+H]+) are then injected into the mass spectrometer
for detection.
Applications
a. APCI is suitable for the analysis of organic compounds with medium – high polarity.
b. Since positive ionization is dependent on protonation, molecules containing basic functional
groups such as amino, amide esters, aldehyde/ketone and hydroxyl can be analyzed.
c. Negative ionization depends upon deprotonation, molecules containing acidic functional
groups are analyzed by this method.
d. Can be used as LC/MS interface.
e. In the analysis of pesticides.
f. Analysis of triazines, phenylureas, carbamates and organophosphorous compounds.
g. In the determination of Vit. D3 in poultry feed supplements.](https://image.slidesharecdn.com/massassingment-190322130213/85/Mass-assingment-13-320.jpg)



![Mass spectroscopy
Assignment1
17
Chemical ionization is carried out in an instrument similar to electron impact ion source
with some modifications such as:-
Addition of a vacuum pump.
Narrowing of exit slit to mass analyzer to maintain reagent gas pressure of about 1 torr in
the ionization chamber.
Providing a gas inlet.
It is a two part process.
Step-I Reagent gas is ionized by Electron Impact ionization in the source. The primary ions of
reagent gas react with additional gas to produce stabilized reagent ions.
Step-II Reagent ions interact with sample molecules to form molecular ions. In this technique
the sample is diluted with a large excess of reagent gas. Gases commonly used as reagent are low
molecular weight compounds such as Methane, tertiary Isobutane, Ammonia, Nitrous oxide,
oxygen and hydrogen etc.
Depending upon the type of ions formed CI is categorized as:-
Positive Chemical Ionization.
Negative Chemical Ionization.
Positive Chemical Ionization
In this technique positive ions of the sample are produced. In positive chemical ionization
gasses such as Methane, Ammonia, Isobutane etc are used
For example Ammonia is used as reagent gas. First ammonia radical cations are generated by
electron impact and this react with neutral ammonia to form ammonium cation (reactive species
of ammonia CI). NH3 → NH3 + + 2 e-
NH3. + → NH4 + + NH2
NH4 + reacts with the sample molecules by proton transfer or Adduct formation to produce
sample ions
M + NH4+ → [M + H] + + NH3 (Proton transfer)
M + NH4+ → [M + NH4]+ (Adduct formation)
When Methane is used as Reagent gas. Methane is ionized by electron impact:
CH4 → CH4+ + 2e-
Primary ions react with additional reagent gas molecules to produce stabilized reagent ions:
CH4+ + CH4 → CH5+ + CH3
CH3+ + CH4 → C2H5+ + H2
The reagent ions then react with the sample molecules to ionize the sample molecules:
CH5+ + MH → CH4 + MH2+ (Proton transfer)
CH3+ + MH → CH4 + M+ (hydride abstraction)
CH4+ + MH → CH4 + MH+ (Charge transfer)
Negative Chemical Ionization
Negative chemical ionization is counterpart of Positive chemical ionization.
Negative ions of the sample are formed and oxygen and Hydrogen are used as reagent gases.
This method is used for ionization of highly electronegative samples. The negative ions are
formed by following reactions:-
Resonance electron capture M + e- → M-
Dissociative electron capture RCl + e- → R + Cl-
H2O + e- → H + OH-](https://image.slidesharecdn.com/massassingment-190322130213/85/Mass-assingment-17-320.jpg)





![Mass spectroscopy
Assignment1
23
A typical (three-dimensional quadrupole) ion trap consists of a cylindrical ring electrode
and two end-cap electrodes. The end-cap electrodes contain holes for the introduction of
ions from an external ion source and for the ejection of ions toward an external detector.
A He bath gas (∼1 mbar) is used to stabilize the ion trajectories in the trap. 74
Benefits:
High sensitivity,
Compactness and mechanical simplicity
Ion/molecule reactions can be studied for mass-selected ions,
High resolution
Non-destructive detection is available using Fourier transform techniques.
Multi-stage mass spectrometry (analogous to FTICR experiments)
Limitations:
Poor quantitation.
Very poor dynamic range (which can be compensated for by employing autoranging).
Collision energy not well-defined in Collision Induced Dissociation [CID] MS/MS.
Quality of the mass spectrum is influenced by many parameters such as excitation, trapping,
and detection conditions.
Mass measurement accuracy is relatively poor.
Applications:
Benchtop GC/MS, LC/MS and MS/MS systems.
Target compound screening.
Ion chemistry.
Non-destructive ion detection
FOURIER-TRANSFORM ION CYCLOTRON
RESONANCE MASS ANALYZER {FTICR-MS/FTMS}
Fourier transform ion cyclotron resonance mass spectrometry is a type of mass analyzer
(or mass spectrometer) for determining the mass-to-charge ratio (m/z) of ions based on
the cyclotron frequency of the ions in a fixed magnetic field.
FT-ICR is the highest performance MS technique available, offering unrivalled resolution
and mass accuracy.](https://image.slidesharecdn.com/massassingment-190322130213/85/Mass-assingment-23-320.jpg)







