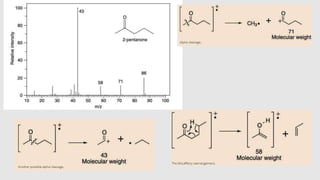
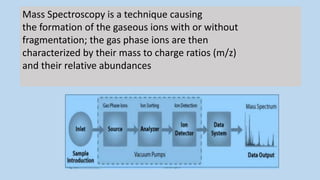
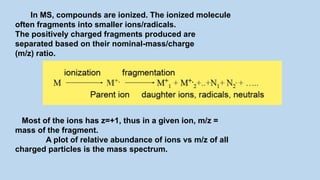
1) Mass spectroscopy involves ionizing compounds and characterizing the resulting ions based on their mass-to-charge ratio.
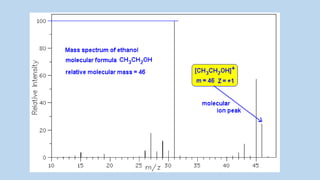
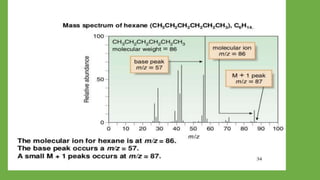
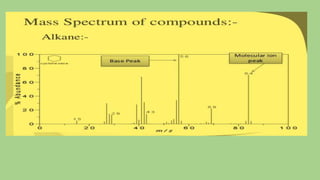
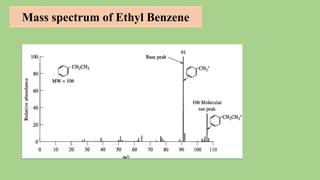
2) When a molecule is bombarded with electrons, it forms a molecular or parent ion by losing an electron.
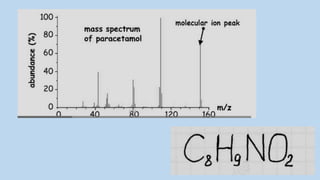
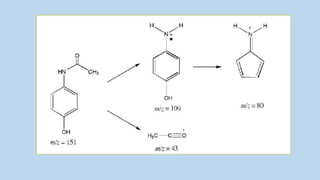
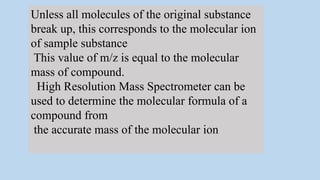
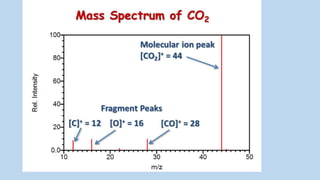
3) The molecular ion peak corresponds to the intact molecule and gives the molecular weight of the compound.




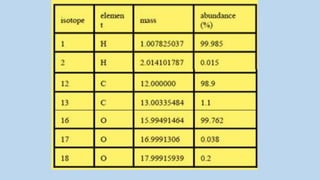
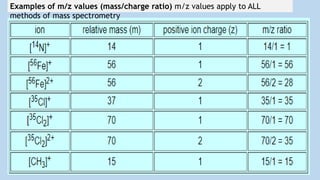
![Chlorine consists of two principal stable isotopes, chlorine-37 (~25% is
37Cl) and chlorine-35 (~75% is 35Cl).
[37Cl37Cl]+ or [37Cl2]+ m/z = 74 (molecular ion)
[35Cl35Cl]+ or [35Cl2]+ m/z = 70 (molecular ion)](https://image.slidesharecdn.com/molecularionpeak-200517165347/85/Molecular-ion-peak-11-320.jpg)