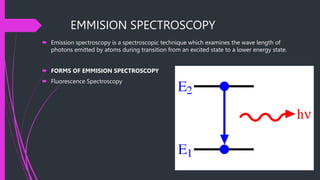
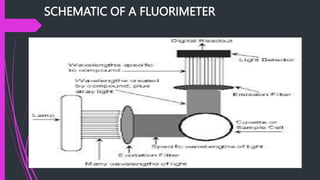
The document provides an overview of fluorescence spectroscopy, detailing its principles, instrumentation, and applications in food analysis. It discusses different types of luminescence, the processes of fluorescence measurement, and specific case studies that demonstrate how this technique can analyze dairy products. Additionally, it highlights the advantages and disadvantages of fluorescence spectroscopy in evaluating food quality and nutritional content.