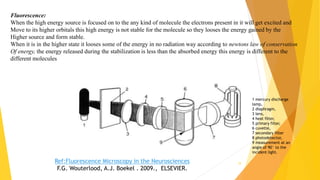
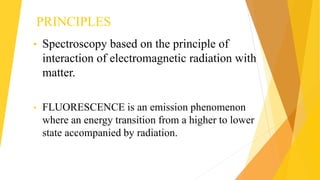
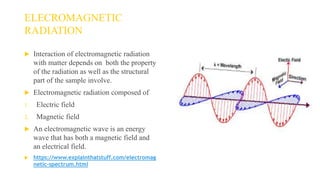
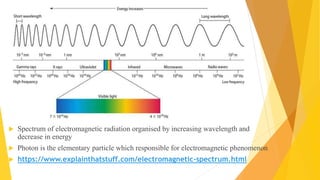
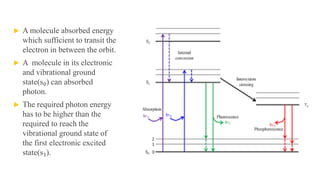
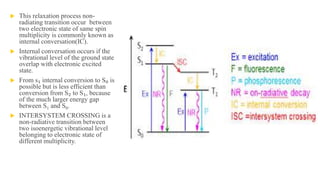
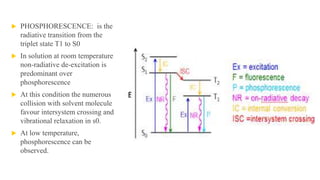
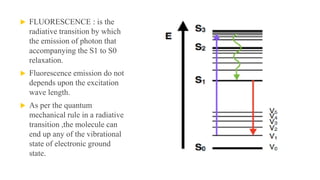
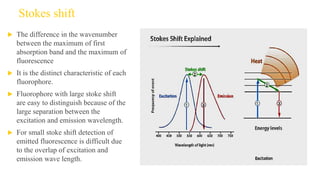
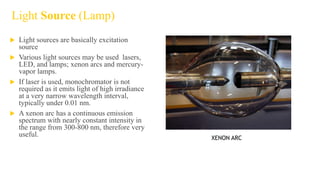
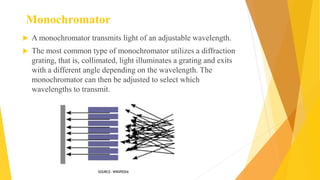
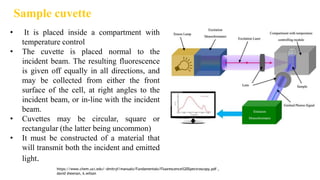
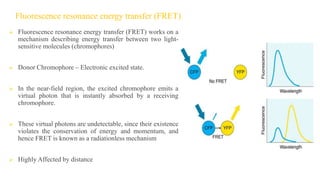
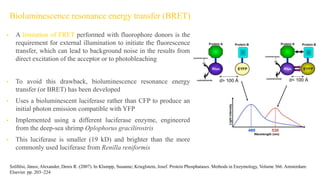
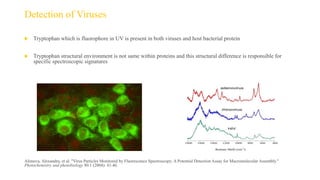
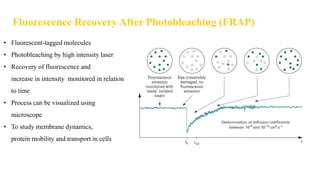
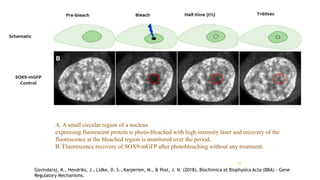
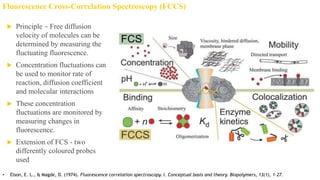
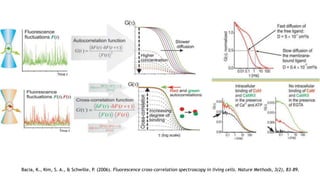
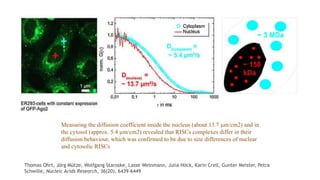
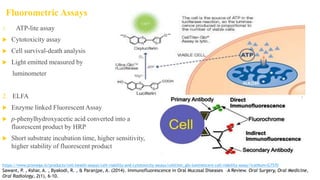
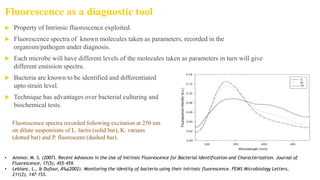
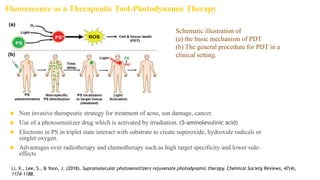
Fluorescence spectroscopy is based on the principle of fluorescence emission that occurs when a molecule absorbs light and is excited to a higher electronic state. The excited molecule then relaxes to the ground state via vibrational relaxation and emission of a photon. The emitted light has a longer wavelength than the absorbed light due to energy losses in vibrational relaxation, following Stokes' rule. Fluorescence spectroscopy can provide information about molecular structure and interactions through analysis of fluorescence emission spectra.