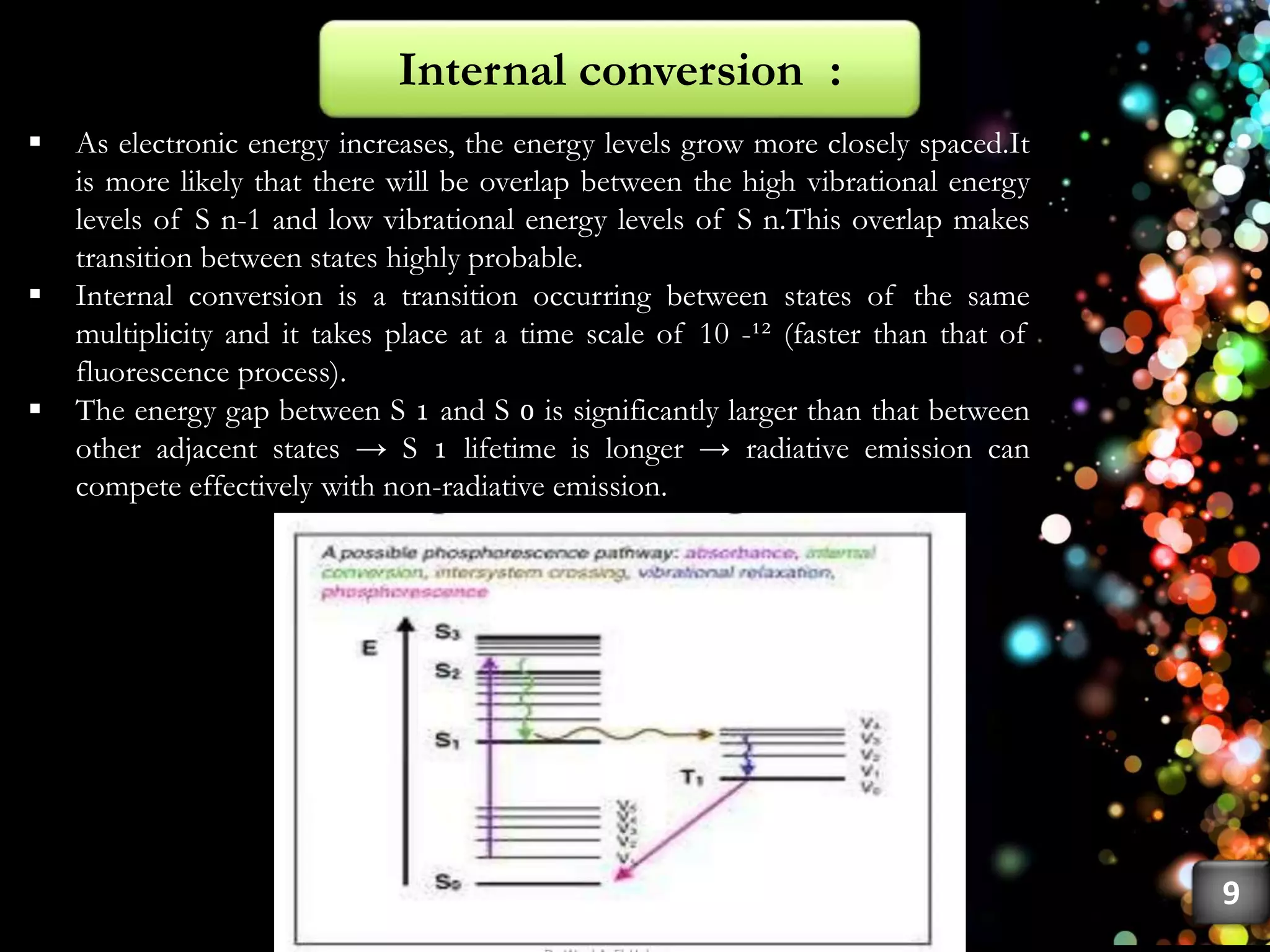
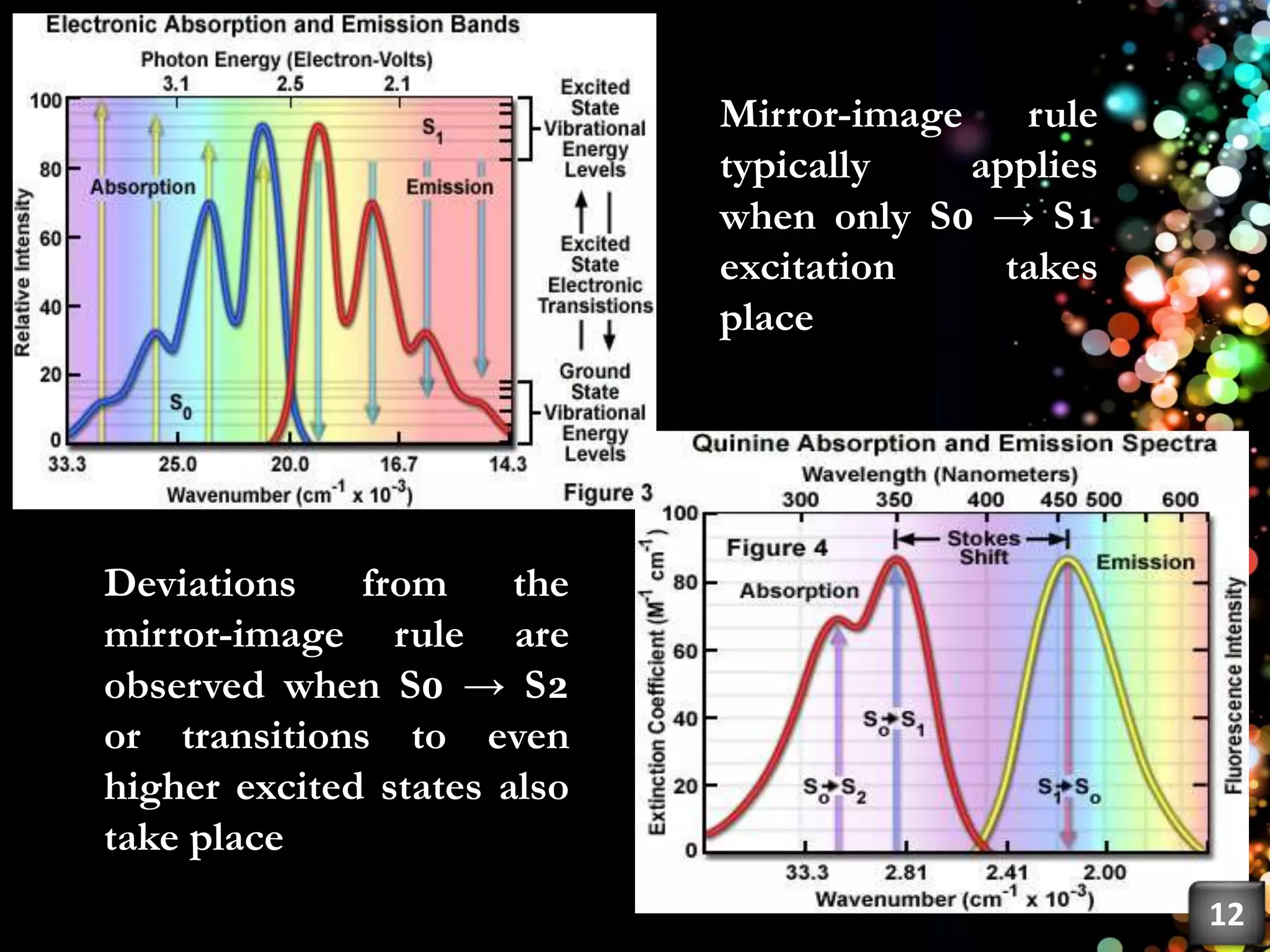
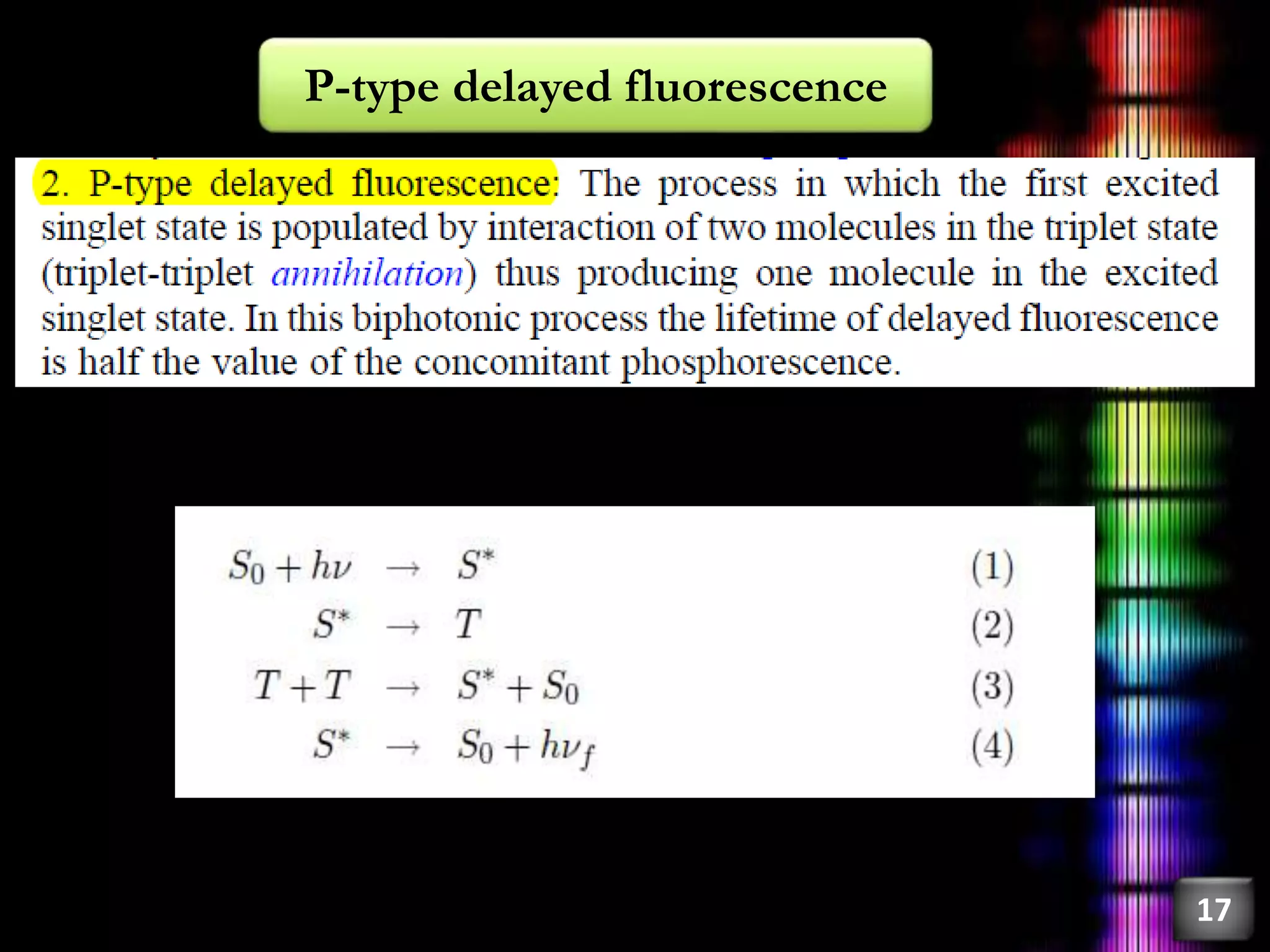
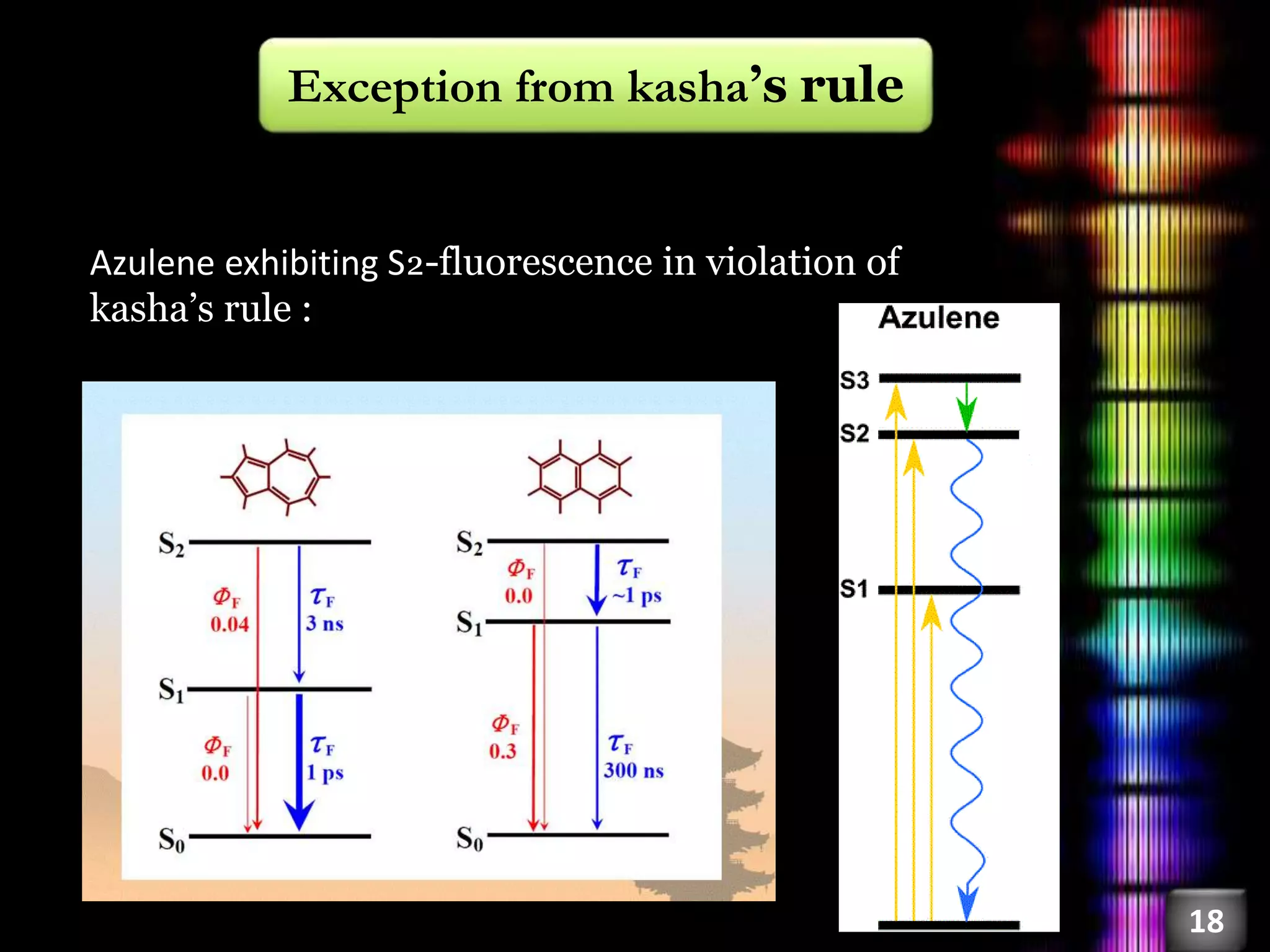
This document discusses fluorescence, including its history, types, principles, and factors that influence it. Fluorescence occurs when a substance emits light as electrons in an excited state return to the ground state. There are three main types: fluorescence spectroscopy, phosphorescence spectroscopy, and chemiluminescence spectroscopy. The principles of fluorescence include Stokes shift, mirror image rule, and exceptions like violations of Kasha's rule. Factors that influence fluorescence intensity include the intrinsic structure of a molecule and its environmental conditions.