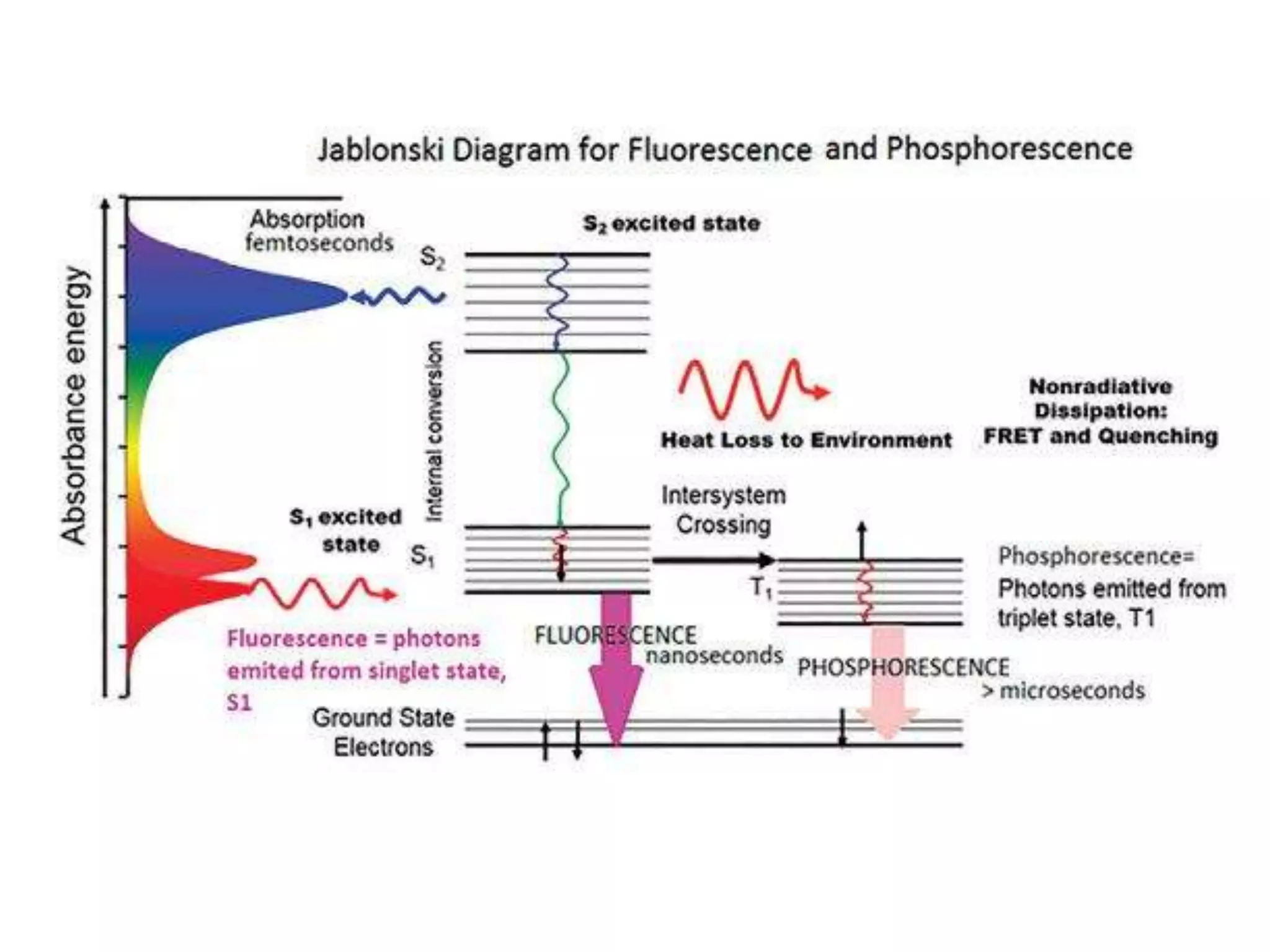
This document discusses different types of luminescence including photoluminescence, chemiluminescence, and electroluminescence. It then focuses on fluorescence spectroscopy, describing how it works, common instrumentation used, and parameters that influence fluorescence spectra such as excitation wavelength and concentration. Applications of fluorescence spectroscopy are outlined as well as techniques like steady-state fluorescence, time-resolved fluorescence, fluorescence anisotropy, and quenching of fluorescence.