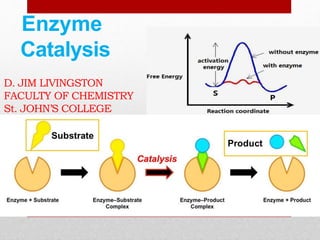
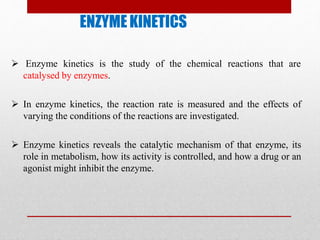
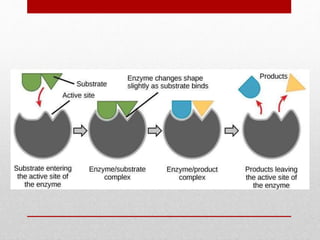
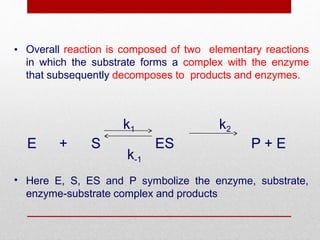
Enzyme kinetics is the study of chemical reactions catalyzed by enzymes. The reaction rate is measured under varying conditions to investigate the catalytic mechanism of the enzyme. The Michaelis-Menten model describes enzyme kinetics as a two-step process where the substrate forms a reversible enzyme-substrate complex that then decomposes into products. This model derives the Michaelis-Menten equation which relates reaction rate to substrate concentration and defines kinetic parameters like the Michaelis constant Km and maximum reaction rate Vmax.

![• According to this model
• When the substrate concentration becomes high enough
to entirely convert the enzyme to the ES form, the second
step of the reaction becomes rate limiting step.
• The general expression of the (rate) of this
reaction is
2
[ E S ]
d t
v
d [ P ]
k](https://image.slidesharecdn.com/enzymecatalysis-200915113059/85/Enzyme-catalysis-6-320.jpg)
![Mechanism
•Now: Based on steady state assumption, d[ES]/dt = 0
• d[ES]/dt = k1[E][S] –k-1[ES] – k2[ES] = 0
•
• d[ES]/dt = k1{[E]0-[ES]}[S] –k-1[ES] – k2[ES] = 0
• k1[E]0[S]-K1[ES][S] –k-1[ES] – k2[ES] = 0
• k1[E]0[S]-K1[ES][S] = k-1[ES] + k2[ES]
• k1[E]0[S] = k-1[ES] + k2[ES]+K1[ES][S]
• k1[E]0[S] = {k-1+ k2+K1[S]}[ES]
• [ES] = [E]0[S] k1/(k-1 + k2+K1[S])
[E]0 [E]+[ES]
[E] [E]0 [ES]
Enzyme conservation equation](https://image.slidesharecdn.com/enzymecatalysis-200915113059/85/Enzyme-catalysis-7-320.jpg)
![Michaelis menten eqn.
K1K2 [E]0[S]
K-1+K2+K1[S]
Dividing by K1,
r = K2 [E]0[S]
(K-1+K2)/K1+[S]
r
K2[E ]0[S]
Km [S ]
r
Rate of the product formation r = K2 [ES]
Km
k1+K2
k1
Km – Michaelis constant](https://image.slidesharecdn.com/enzymecatalysis-200915113059/85/Enzyme-catalysis-8-320.jpg)
![Plot of [S] vs rate
If [E]0 = [ES]
Then,
rmax= K2[E]0 = Vmax
Sub in MM eqn,
r = Vmax[S]
Km+[S]
K2[E ]0[S]
Km [S ]
Rate of the product formation r = K2 [ES]](https://image.slidesharecdn.com/enzymecatalysis-200915113059/85/Enzyme-catalysis-9-320.jpg)
![If Km = [S]
Plot of [S] vs rate](https://image.slidesharecdn.com/enzymecatalysis-200915113059/85/Enzyme-catalysis-10-320.jpg)
![MICHAELIS - MENTEN CONSTANT
• It is the substrate concentration, [S] at which half maximum
velocity of reaction is observed.
• K m implies that half of the active sites on the enzymes arefilled.
• It is the measure of the binding strength between substrate and the
enzyme. (lower K m value indicates a strong affinity betweenthe
substrate and enzymes.
40 M in 2 day
40 M in 12 days](https://image.slidesharecdn.com/enzymecatalysis-200915113059/85/Enzyme-catalysis-11-320.jpg)
![Plot of [S] vs rate
Case – I:
If Km >>>> [S]
Linear plot
Case – II
If [S] >>>> Km
Plateau region
First order
Zero order](https://image.slidesharecdn.com/enzymecatalysis-200915113059/85/Enzyme-catalysis-12-320.jpg)
![Vmax
It is the maximum velocity of reaction or rate at which
the enzyme catalyzed a reaction under given conditions.
Vmax is reached when all enzyme sites are saturated with the
substrate.
This will happen when substrate concentration [S] is greater than
K m.](https://image.slidesharecdn.com/enzymecatalysis-200915113059/85/Enzyme-catalysis-13-320.jpg)
