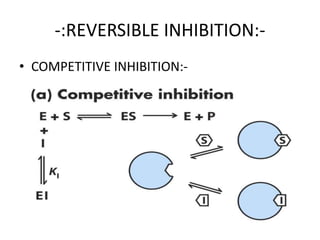
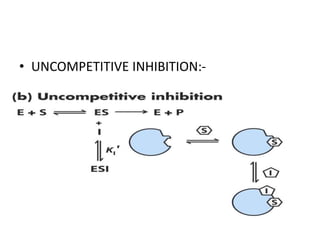
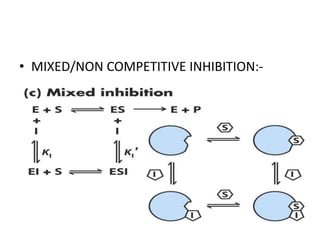
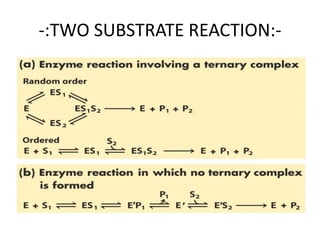
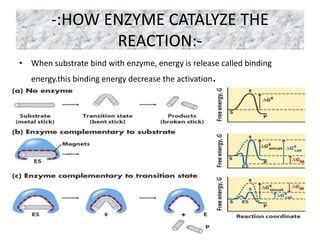
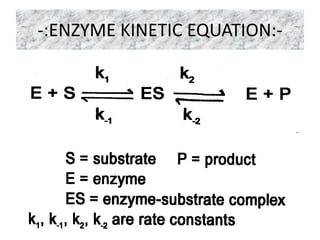
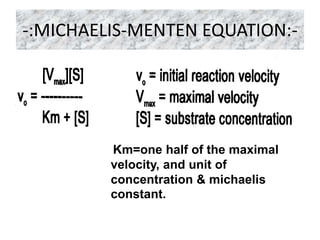
The document provides an overview of enzyme kinetics, detailing the structure of enzymes and their catalytic function. It discusses key concepts such as the Michaelis-Menten equation, its derivation, and the effects of reversible inhibition in enzymatic reactions. The conclusion emphasizes the significance of enzyme kinetics in understanding biological catalysts and their role in transforming substrates into products.


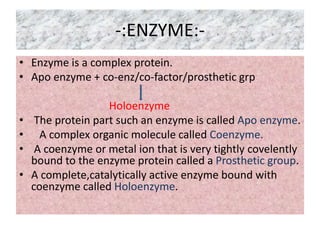
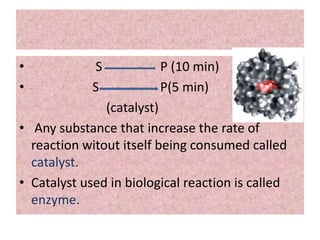


![-:MICHAELIS-MENTEN EQUATION
DERIVATION:-
• E+S ES EP E+P (1)
• (2)
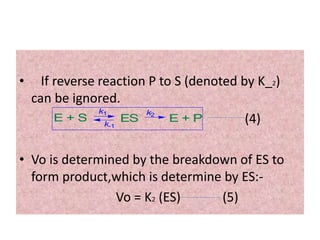
• Michaelis and Menten derived this equation starting from their basic
hypothesis that the rate-limiting step in enzymatic reactions is the
breakdown of the ES complex to product and free enzme. The important
terms are [S], Vo, Vmax, and a constant called the Michaelis constant.
•
• (3)](https://image.slidesharecdn.com/enzymekinectics-200515081030/85/Enzyme-kinectics-by-kk-sahu-11-320.jpg)
![• Rate of ES formation = k1([ET] - [ES])[S]
(where [ET] is total concentration of
enzyme E and k-2 is considered
neglible)
• Rate of ES breakdown to product = k-
1[ES] + k2[ES]](https://image.slidesharecdn.com/enzymekinectics-200515081030/85/Enzyme-kinectics-by-kk-sahu-13-320.jpg)
![k1([ET] - [ES])[S] = k-1[ES] + k2[ES] (6)
Rate of formation of ES is equal to the rate
of its breakdown. This is called “Steady-
state assumption.”](https://image.slidesharecdn.com/enzymekinectics-200515081030/85/Enzyme-kinectics-by-kk-sahu-14-320.jpg)
![• k1[ET][S]-k1[ES][S]=[k-1 + k2] [ES] (7)
Left side is multiplied out and the right side
simplified given.](https://image.slidesharecdn.com/enzymekinectics-200515081030/85/Enzyme-kinectics-by-kk-sahu-15-320.jpg)
![• k1[ET][S]=(k1 [S]+ k-1 + k2 )[ES] (8)
• We solve the equation for[ES]
k1[ET][S]
[ES]= (9)
k1 [S]+ k-1 + k2](https://image.slidesharecdn.com/enzymekinectics-200515081030/85/Enzyme-kinectics-by-kk-sahu-16-320.jpg)
![[ET][S]
• [ES]= (10)
[S]+ (k-1 + k2 )/ k1](https://image.slidesharecdn.com/enzymekinectics-200515081030/85/Enzyme-kinectics-by-kk-sahu-17-320.jpg)
![[ET][S]
• [ES] = (11)
Km + [S]
The term (k-1 + k2 )/ k1 is defined as the
Michaelis constant, Km.](https://image.slidesharecdn.com/enzymekinectics-200515081030/85/Enzyme-kinectics-by-kk-sahu-18-320.jpg)
![k2 [ET][S]
• Vo = (12)
Km + [S]
Here Vo = k2 [ ES ] in the equation 5.](https://image.slidesharecdn.com/enzymekinectics-200515081030/85/Enzyme-kinectics-by-kk-sahu-19-320.jpg)
![• (13)
• Maximum velocity occurs when the enzyme is
saturated(that is [ES]= [ ET ])Vmax can be
defined as k2 (ET ).This is Michaelis-
Menten equation.](https://image.slidesharecdn.com/enzymekinectics-200515081030/85/Enzyme-kinectics-by-kk-sahu-20-320.jpg)
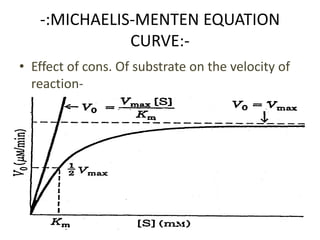
![Vmax Vmax [S]
= (14)
2 Km + [S]
Vo is exactly one-half Vmax .](https://image.slidesharecdn.com/enzymekinectics-200515081030/85/Enzyme-kinectics-by-kk-sahu-21-320.jpg)
![• 1 [S]
= (15)
2 Km + [S]
on dividing by Vmax .](https://image.slidesharecdn.com/enzymekinectics-200515081030/85/Enzyme-kinectics-by-kk-sahu-22-320.jpg)
![ solving for Km we get Km +S=2S
or
• Km =[S], when Vo = 1/2 Vmax.](https://image.slidesharecdn.com/enzymekinectics-200515081030/85/Enzyme-kinectics-by-kk-sahu-23-320.jpg)