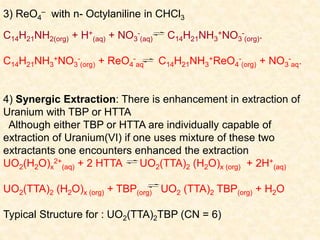
This document discusses solvent extraction, which is a versatile separation method used in analytical chemistry. It can be used to separate, purify, enrich, and analyze both tracer and macro amounts of metal ions. The key principles discussed include the phase rule, which describes solvent extraction as a two-phase system, and the Nernst distribution law, which defines the partition or distribution coefficient. Different types of extraction systems are classified, including chelate extraction involving complex formation, extraction by solvation, and ion-pair formation. Factors that affect metal complex stability such as ligand basicity and ring size are also outlined.




![According to Nernst distribution law if [X1] is concentration of
solute in phase 1 and if [X2] is the concentration of solute in phase
2 at equilibrium X1, X2
i.e [X2]
KD = --------------- KD = Partition coefficient
[X1]
This partition or distribution coefficient is independent of the
total solute concentration in either of the phases. In the above
expression for KD. We have not considered the activity coefficient
of the species in the organic as well as in the aqueous phase.
We therefore use the term distribution ratio (D) to account for
the total concentration of the species in two phases. In the
circumstances we have distribution ratio (D) as
Total concentration of species in the organic phase
D= -------------------------------------------------------------------------
Total concentration of species in the aqueous phase](https://image.slidesharecdn.com/solventextraction-210129004244/85/Solvent-extraction-5-320.jpg)
![Now assuming if there is no association dissociation or
polymerization in the phase
Then under the idealized condition KD = D
one prefers to use term percentage extraction (%E) . This is
related to distribution ratio (D) by an expression as
(Vw / Vo ) . E Vw = Vol. of aq. Phase.
D = -------------------- Vo = Vol. of org. Phase.
(100 – E)
When volume of organic and aqueous phases are equal i.e.
Vo = Vw , D reduces to
D = [E / (100 – E)]
Further the extraction is considered to be quantitative
when E = 100
D = [100 / 100 – 100]
= 100 / 0 = ∞ ( if Vo = Vw )](https://image.slidesharecdn.com/solventextraction-210129004244/85/Solvent-extraction-6-320.jpg)




![2) Extraction by solvation : The extracted species
gets solvated into the organic phase. e.g.
Extraction of Iron (III) from 6M HCl with diethyl
ether
• Fe 3+ + 4 Cl- FeCl4
-
• FeCl4
- + H+ [H+, FeCl4
- ]
• {(C2H5)2O : H+, FeCl4 [(C2H5)2O]2
-
• Oxygen atom of the solvent molecule coordinate
with metal ion – oxonium extraction system.](https://image.slidesharecdn.com/solventextraction-210129004244/85/Solvent-extraction-11-320.jpg)

![3) Ion - pair formation : The extraction proceed with the
formation of neutral unchanged species gets extracted into
organic phase.
Mn+ + b B MBb
n+
MBb
n+ + nX- (MBb
n+, nX- ) cationic complex
Where B = Neutral ligand X = anion M = metal
Mn+ + (n-a)X- Mxa-
n+a
Mxa-
n+a+ aY+ (aY+, MXa-
n+a) anionic complex
e.g. 1) Extraction of Cu(II) with 1, 10- phenanthroline in CHCl3.
[Cu (Phen)2
+; 2ClO4
-] Cationic complex.
2) Iron (III) with ether is anionic type.
[H(ether)+, FeCl4 (ether)-]](https://image.slidesharecdn.com/solventextraction-210129004244/85/Solvent-extraction-13-320.jpg)