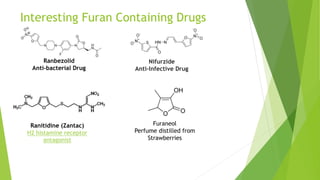
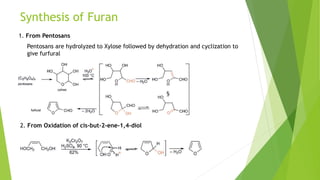
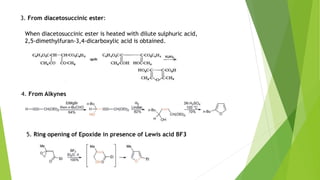
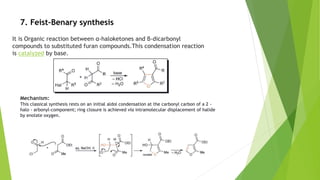
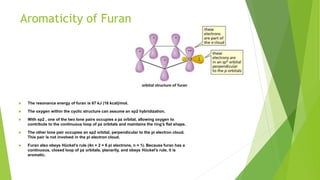
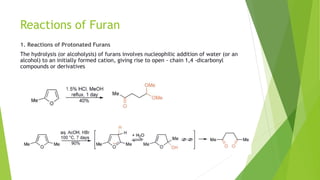
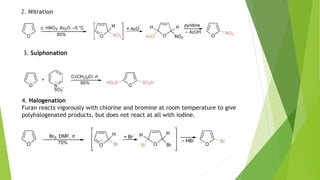
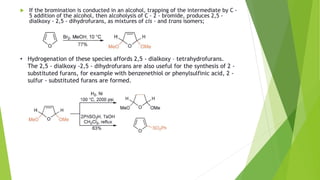
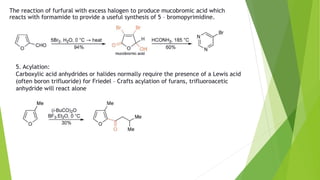
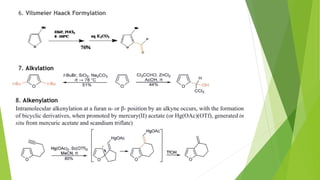
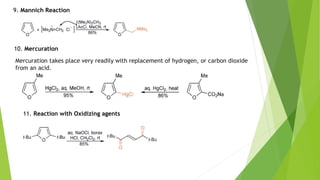
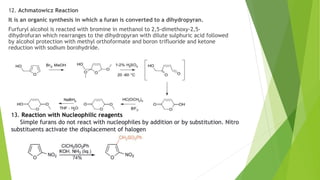
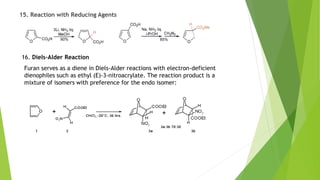
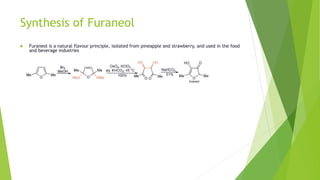
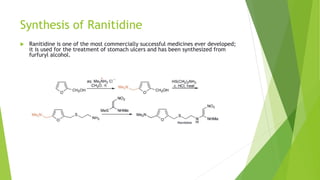
Furan is a heterocyclic organic compound consisting of a five-membered aromatic ring with four carbon atoms and one oxygen atom. It is a colorless, flammable, and highly volatile liquid. Some important drugs containing furan rings include ranbezolid, nifurzide, and ranitidine. Furan can be synthesized through several methods, such as from pentosans, oxidation of cis-but-2-ene-1,4-diol, and from diacetosuccinic ester. It undergoes various reactions including electrophilic substitution, nitration, sulfonation, halogenation, acylation, and Diels-Alder reactions. Furaneol and ran