Embed presentation
Download to read offline
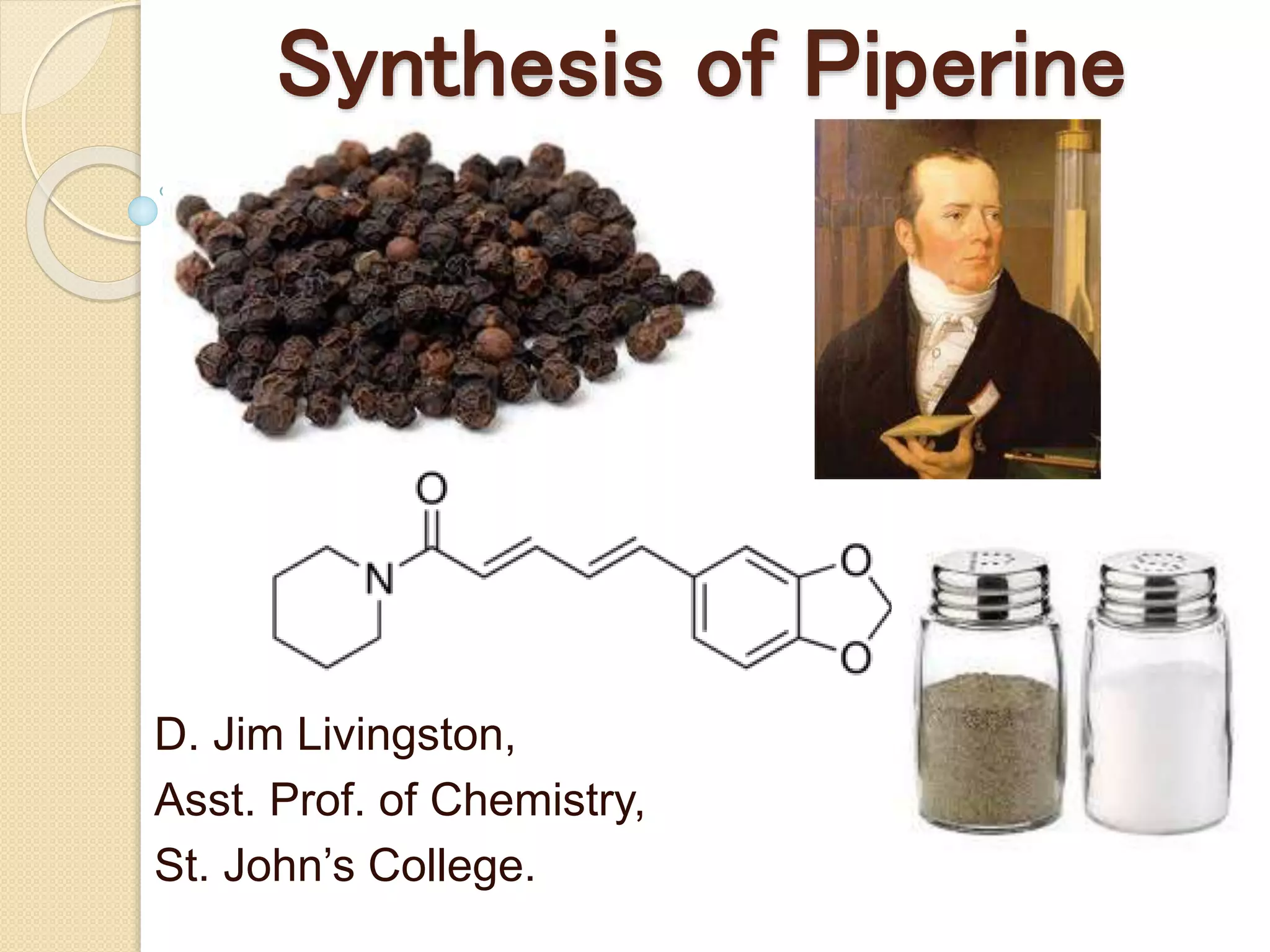

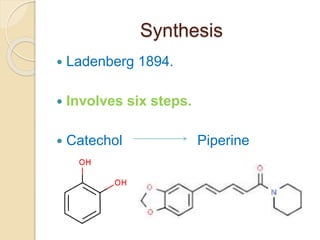
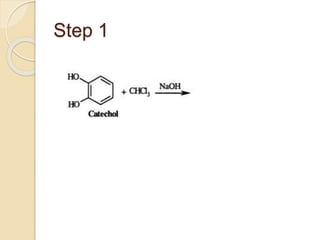
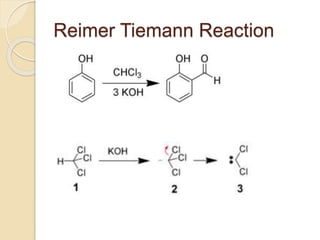
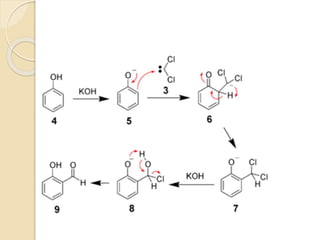
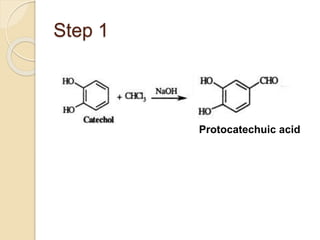
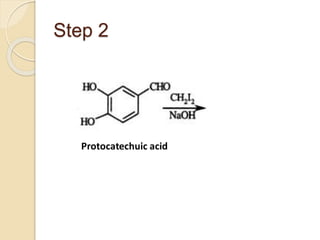
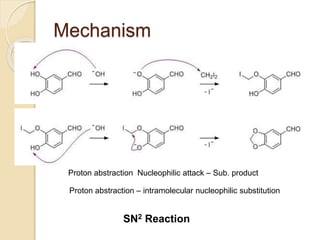
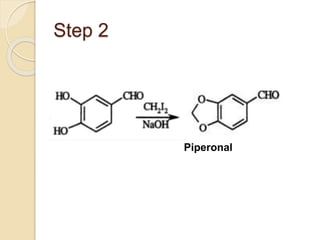
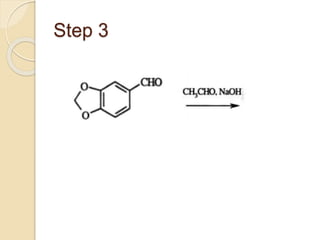
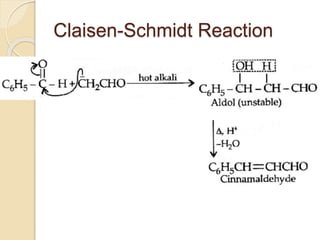
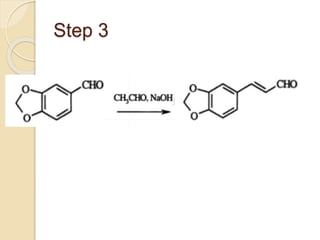
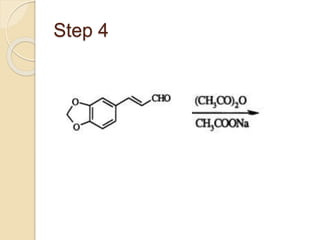
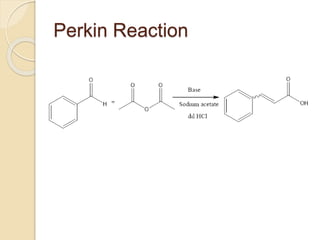
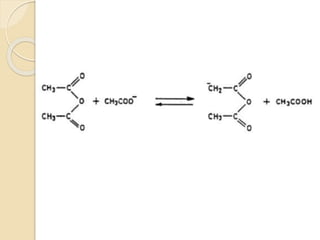
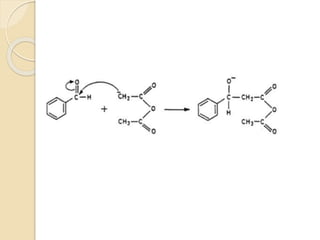
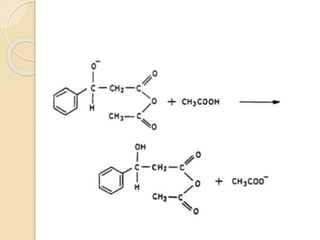
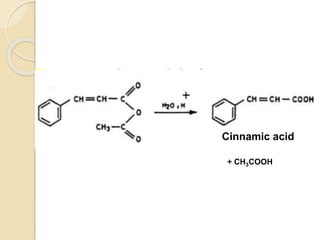
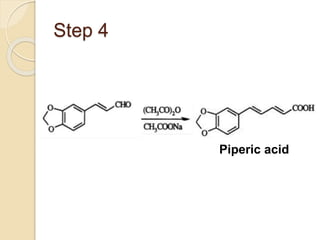
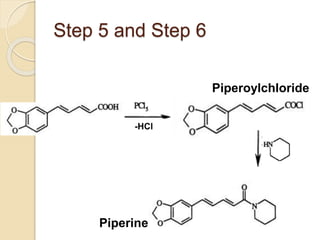
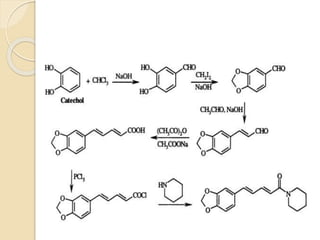



Piperine is a yellow crystalline compound that is slightly soluble in water but more soluble in alcohol. It forms salts with strong acids and has properties of melting point between 128 to 130 °C. Piperine is used as a food spice and flavoring agent that can increase nutrient absorption and improve attention and reasoning skills. The six step synthesis of piperine was developed in 1894 and involves the Reimer-Tiemann reaction, Claisen-Schmidt reaction, Perkin reaction, and acid-catalyzed reactions to convert protocatechuic acid to piperine.























