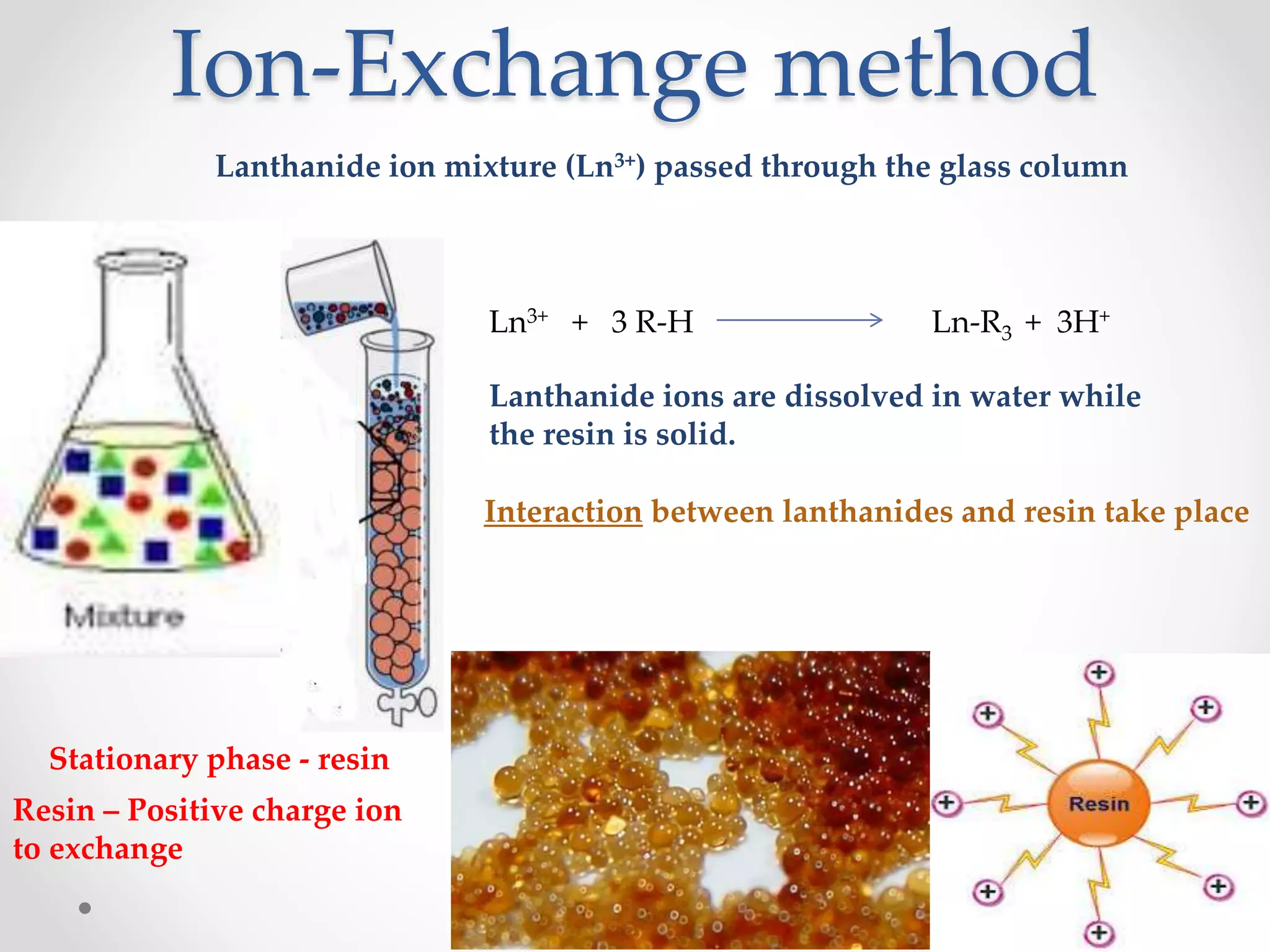
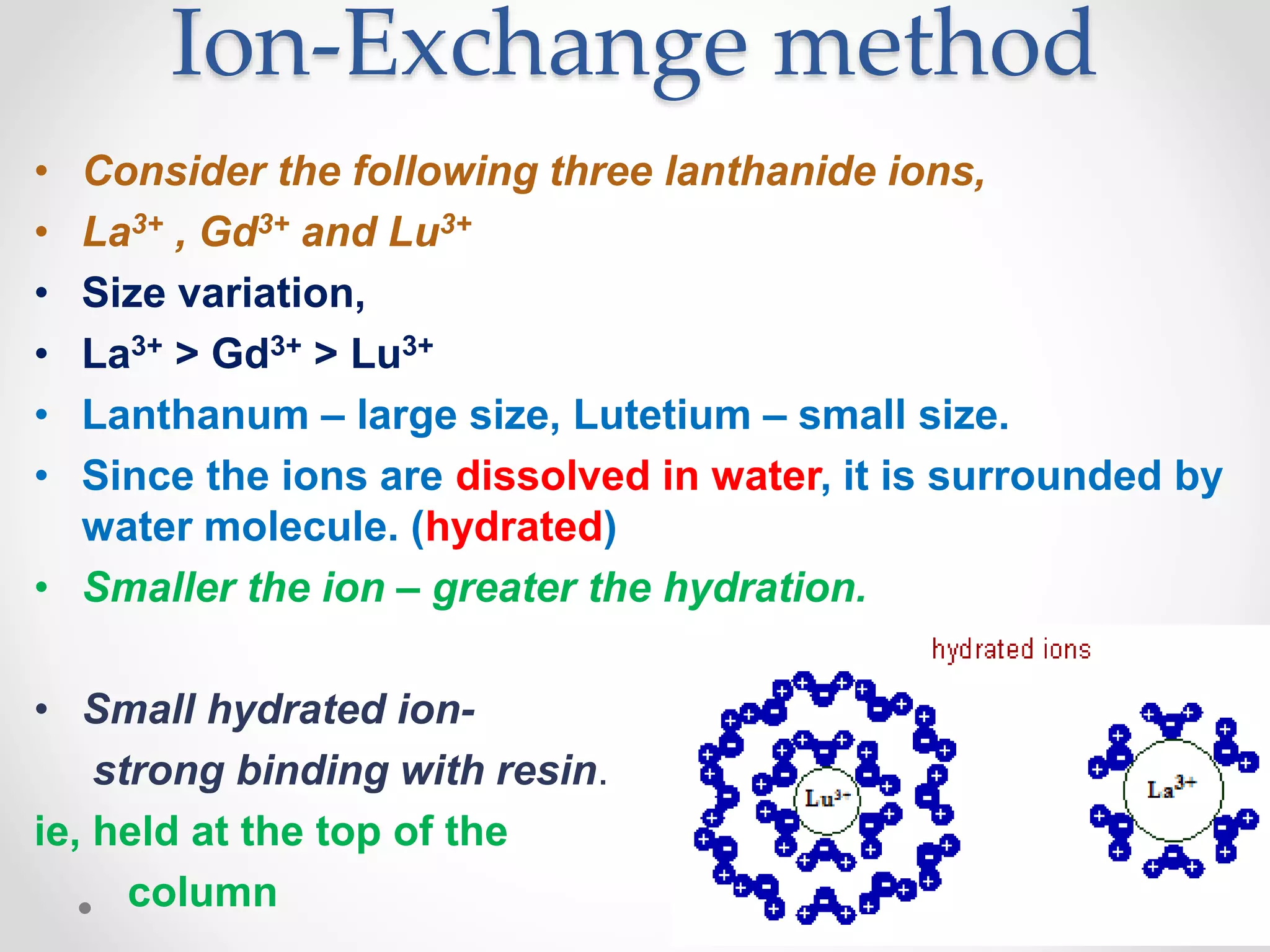
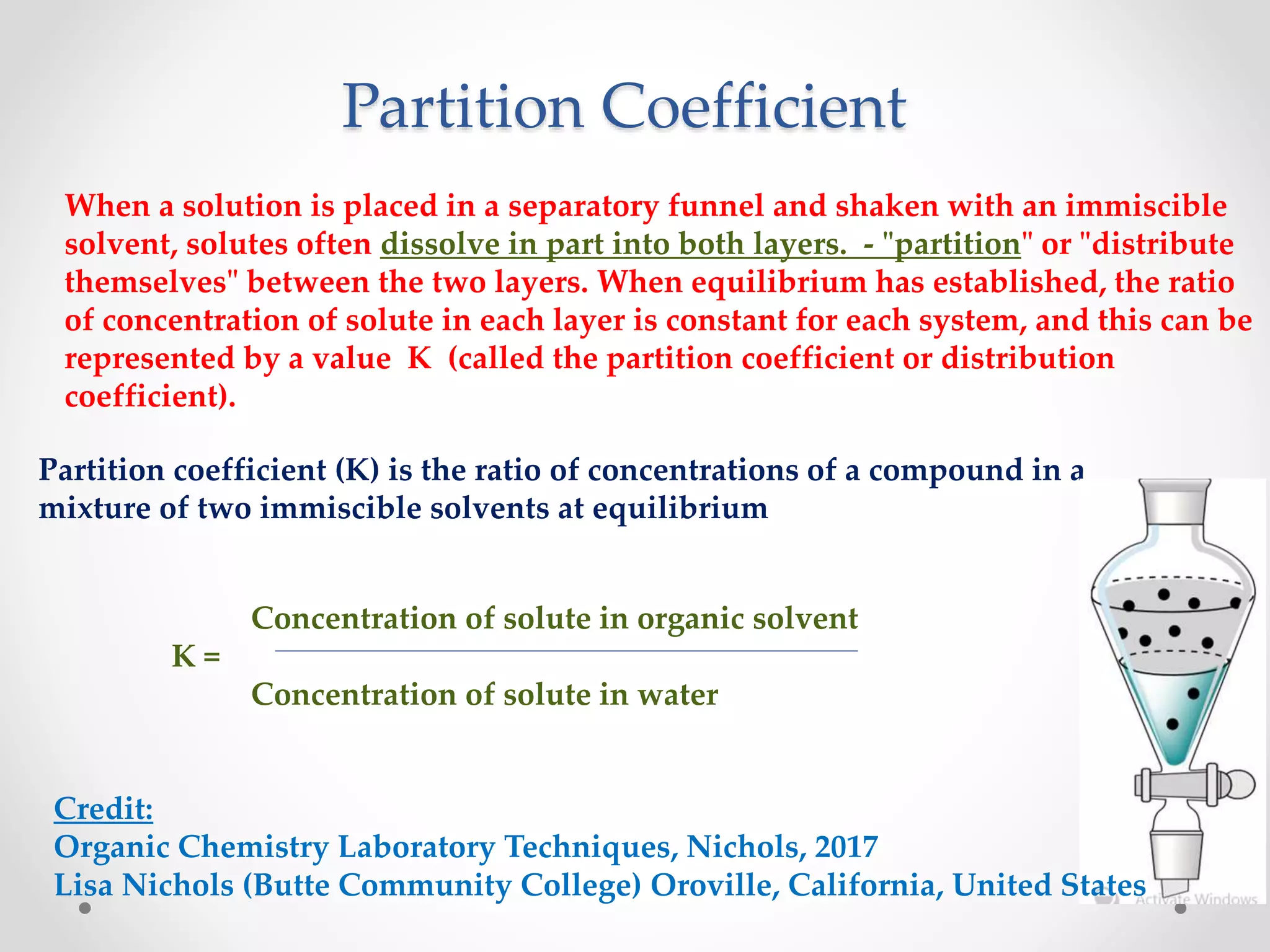
This document discusses methods for separating lanthanide ions using ion exchange and solvent extraction. In ion exchange, lanthanide ions in solution are passed through a resin column and interact differently with the resin based on their size, allowing for separation. Smaller ions interact more strongly and elute first. Solvent extraction involves dissolving lanthanide ions in water and adding an immiscible organic solvent. The partition coefficient determines how ions distribute between the two solvents, allowing separation based on differences in this value for different ions through multiple extractions.