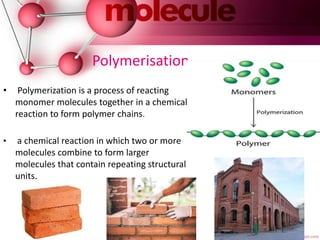
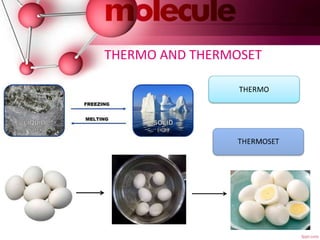
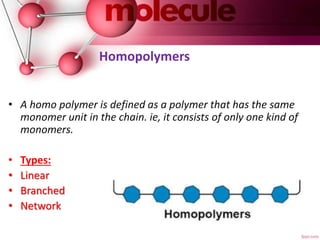
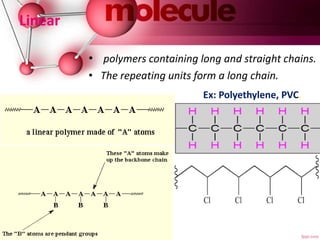
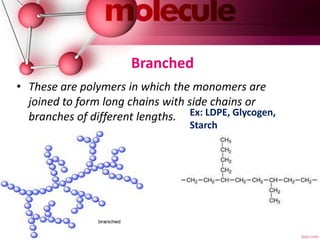
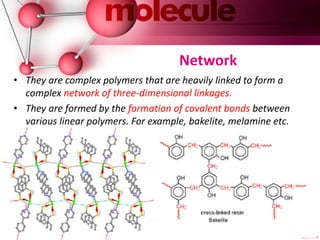
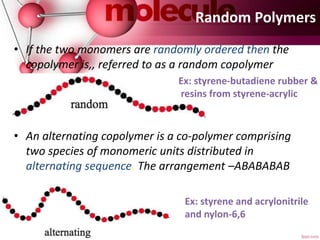
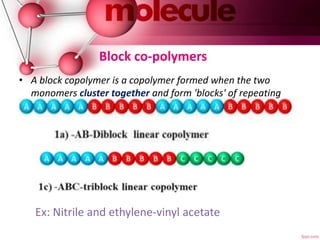
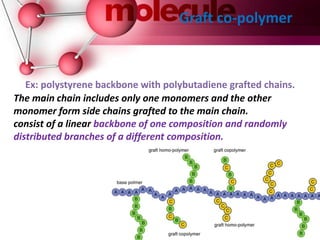
This document provides an introduction to polymer chemistry. It defines key terms like polymer, monomer, and polymerization. Polymers can be classified in several ways, including by origin (natural, synthetic, semi-synthetic), properties/applications (rubbers, plastics, fibers), thermal response (thermoplastics, thermosets), and monomer structure (homopolymers, co-polymers like random, alternating, block, and graft). Common examples are given for each classification.