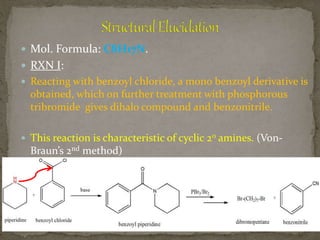
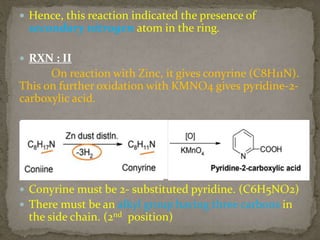
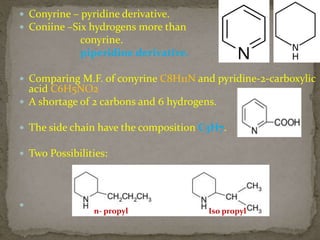
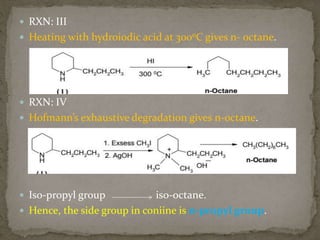
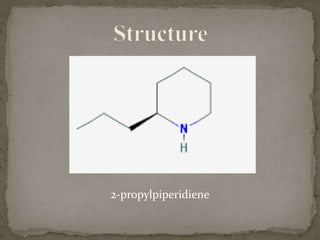
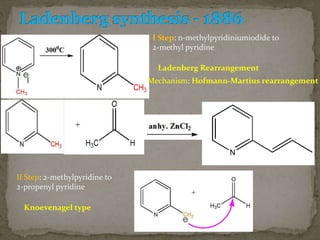
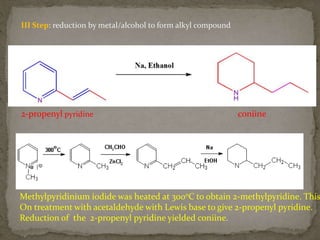
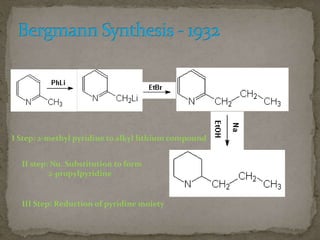
This document describes the chemical structure and synthesis of coniine. Coniine is a highly toxic piperidine alkaloid obtained from hemlock plants. The document outlines reactions that determined coniine has the molecular formula C8H17N and is a 2-propylpiperidine. It also describes three historical syntheses of coniine: the Ladenburg synthesis from 1886, the Bergmann synthesis from 1932, and a more recent nucleophilic substitution synthesis. Coniine was famously used to execute the Greek philosopher Socrates in 399 BC and is still used in some countries to carry out capital punishment.