This document discusses microwave spectroscopy and its application to determining properties of gas phase molecules. It can be summarized as follows:
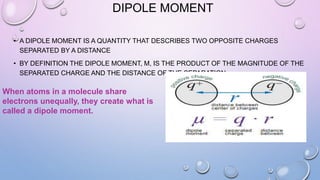
1) Microwave spectroscopy utilizes photons in the microwave range to cause rotational energy level transitions in gas molecules. It is applicable to molecules with a permanent dipole moment in the gas phase.
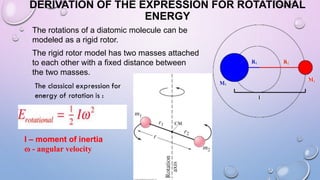
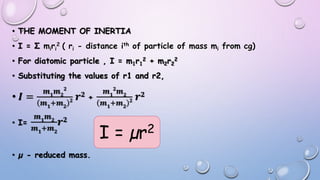
2) The rotational energy levels of diatomic molecules can be modeled using a rigid rotor approximation. This allows derivation of an expression for rotational energy levels in terms of the rotational constant B, which depends on the molecule's moment of inertia.
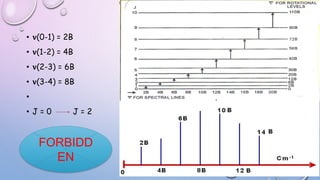
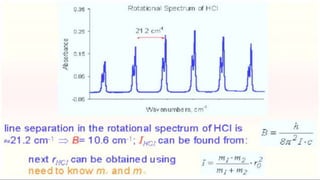
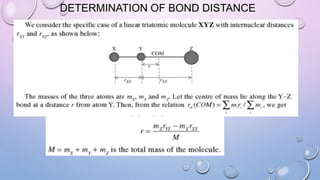
3) Measurement of transition frequencies between rotational energy levels allows determination of the rotational constant B. This can then be used to calculate bond distances in diatomic molecules.