The document provides information on the International Conference on Harmonization (ICH), including:
- ICH aims to harmonize technical requirements for pharmaceutical registration across regions to ensure safety and efficacy.
- It involves regulators and industry from the EU, Japan, and USA.
- The goals are to establish common guidelines and make information available globally.
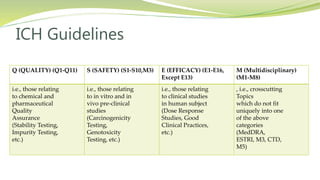
- ICH guidelines cover quality, safety, efficacy, and multidisciplinary topics for drug development and review.
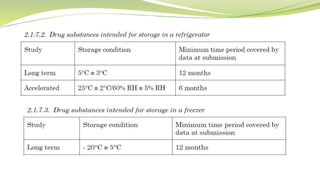
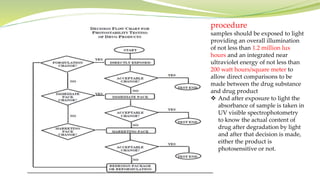
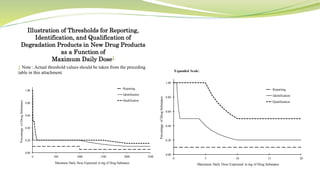
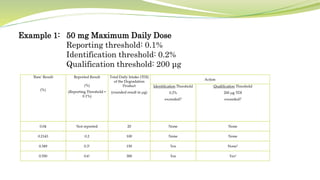
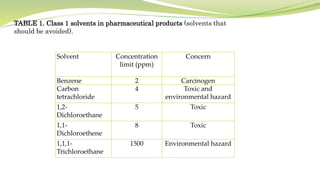
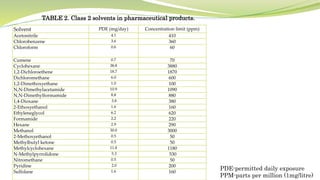
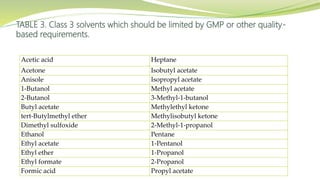
- The document then focuses on specific ICH guidelines related to quality, including stability testing, analytical method validation, and impurities.