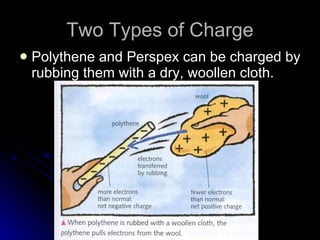
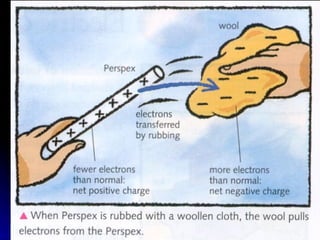
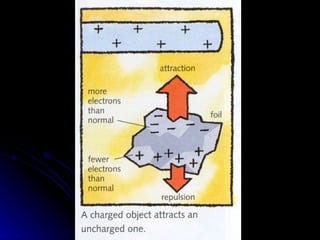
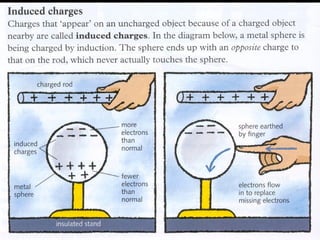
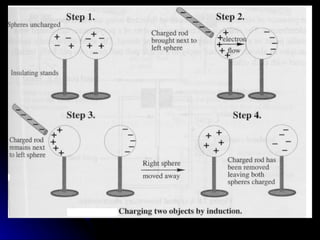
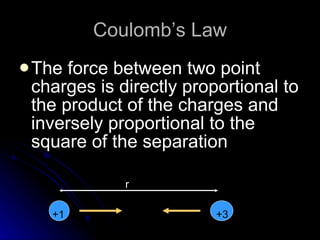
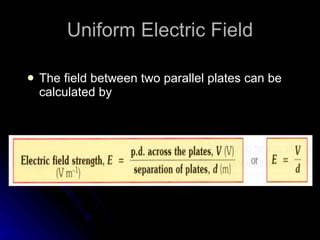
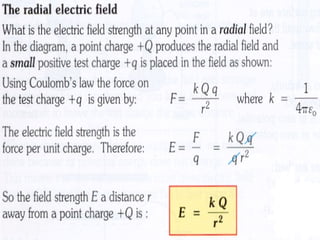
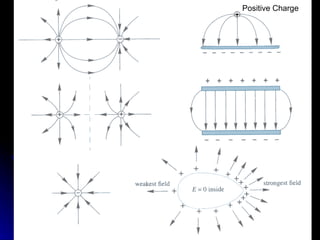
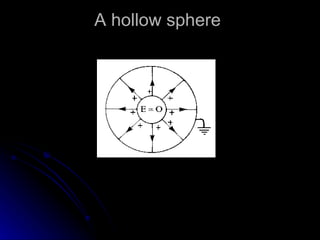
This document discusses electric fields and forces. It explains that electric charge comes from electrons and protons in atoms, and that rubbing materials together can transfer electrons, creating a separation of positive and negative charges. It also describes how conductors, insulators, and semiconductors differ in how easily they allow charge to flow through or remain separated.