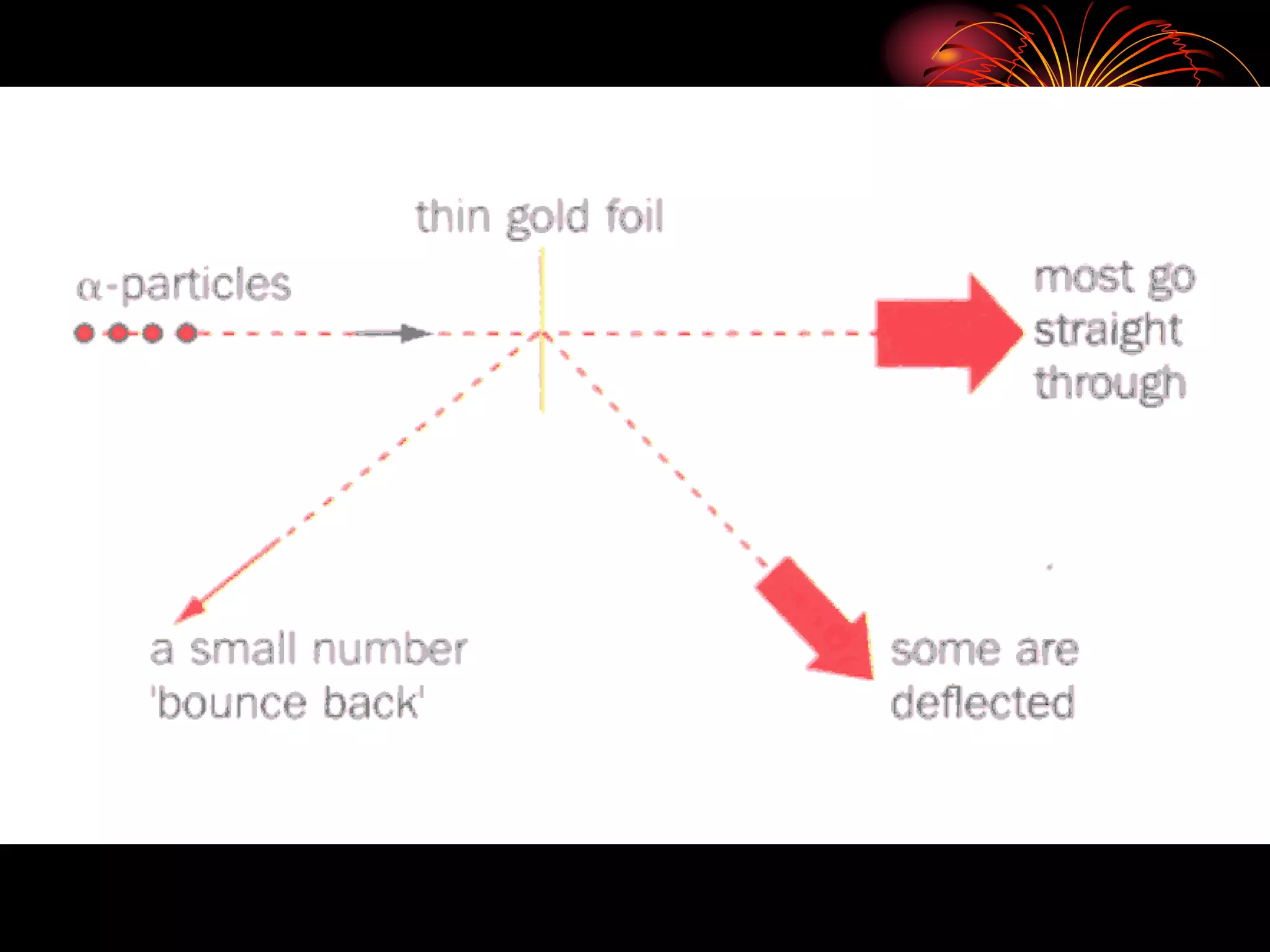
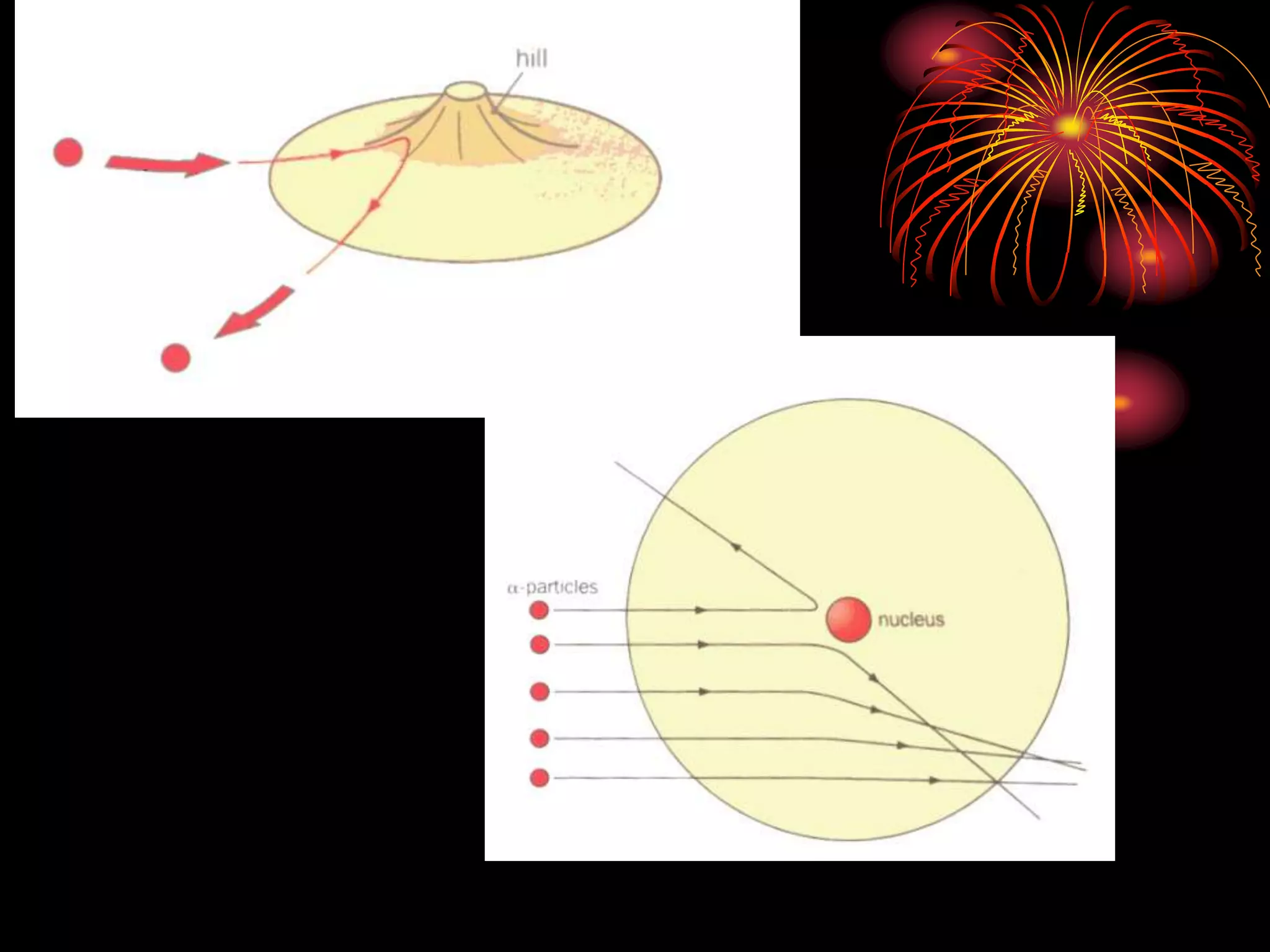
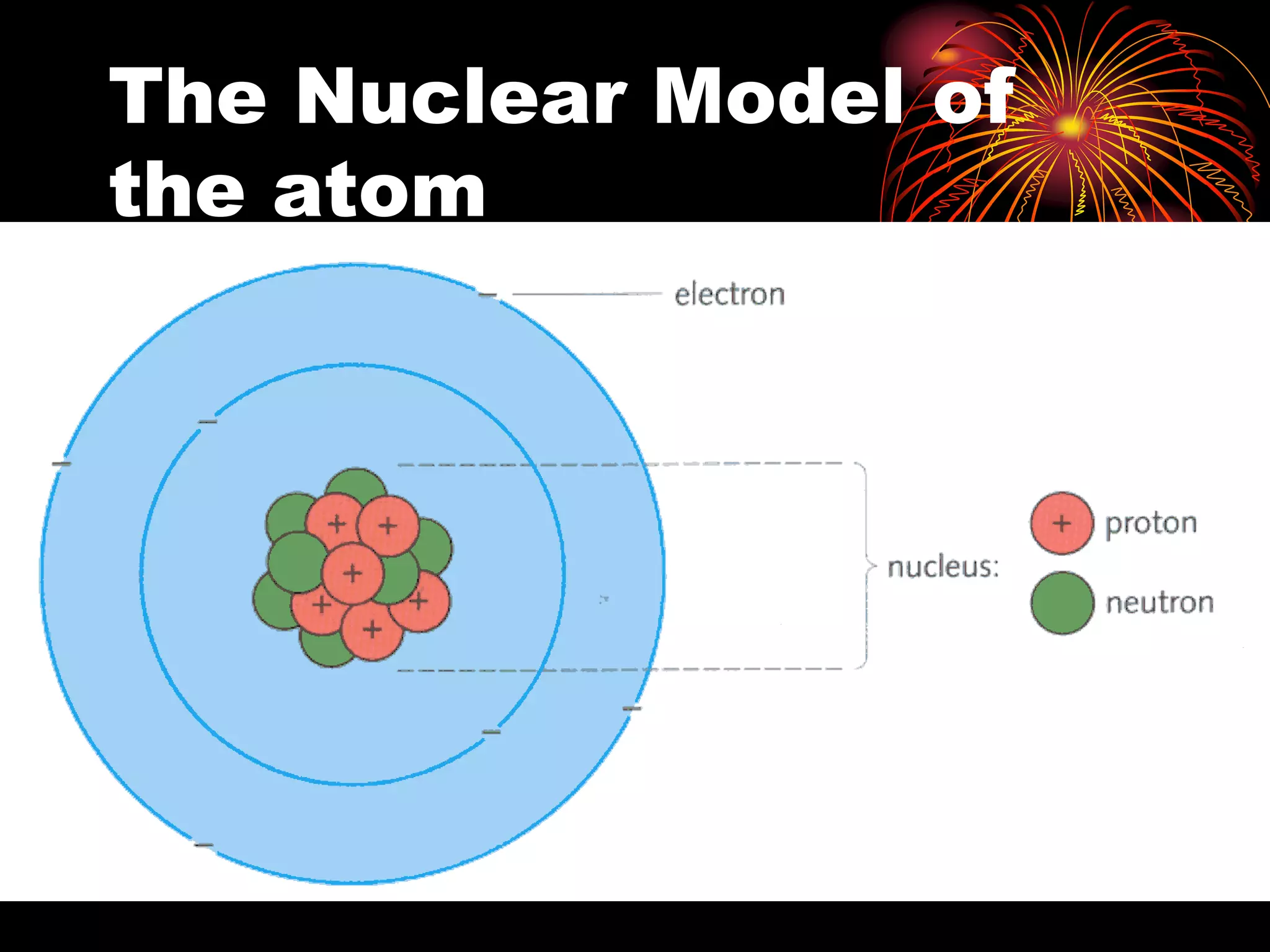
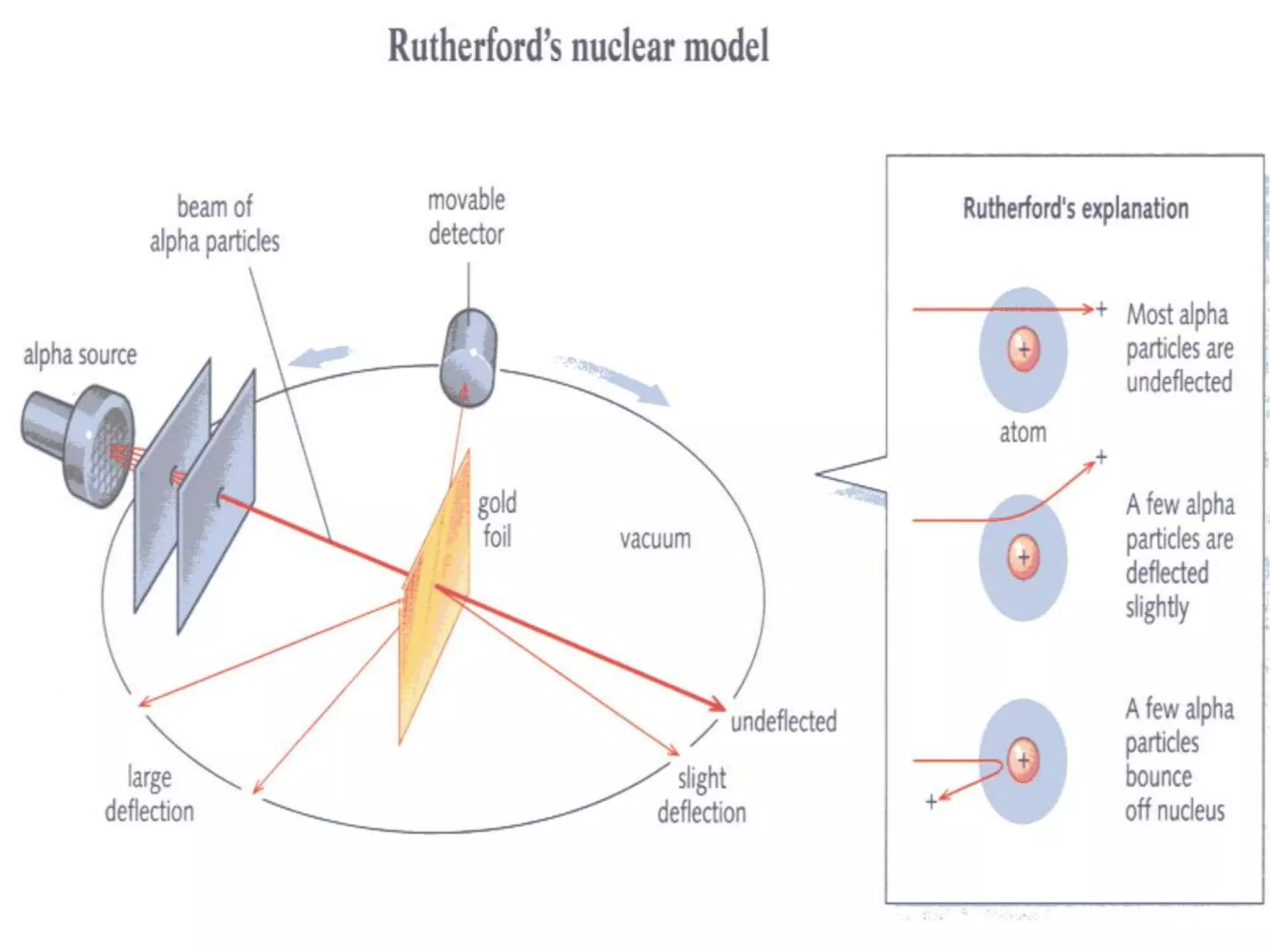
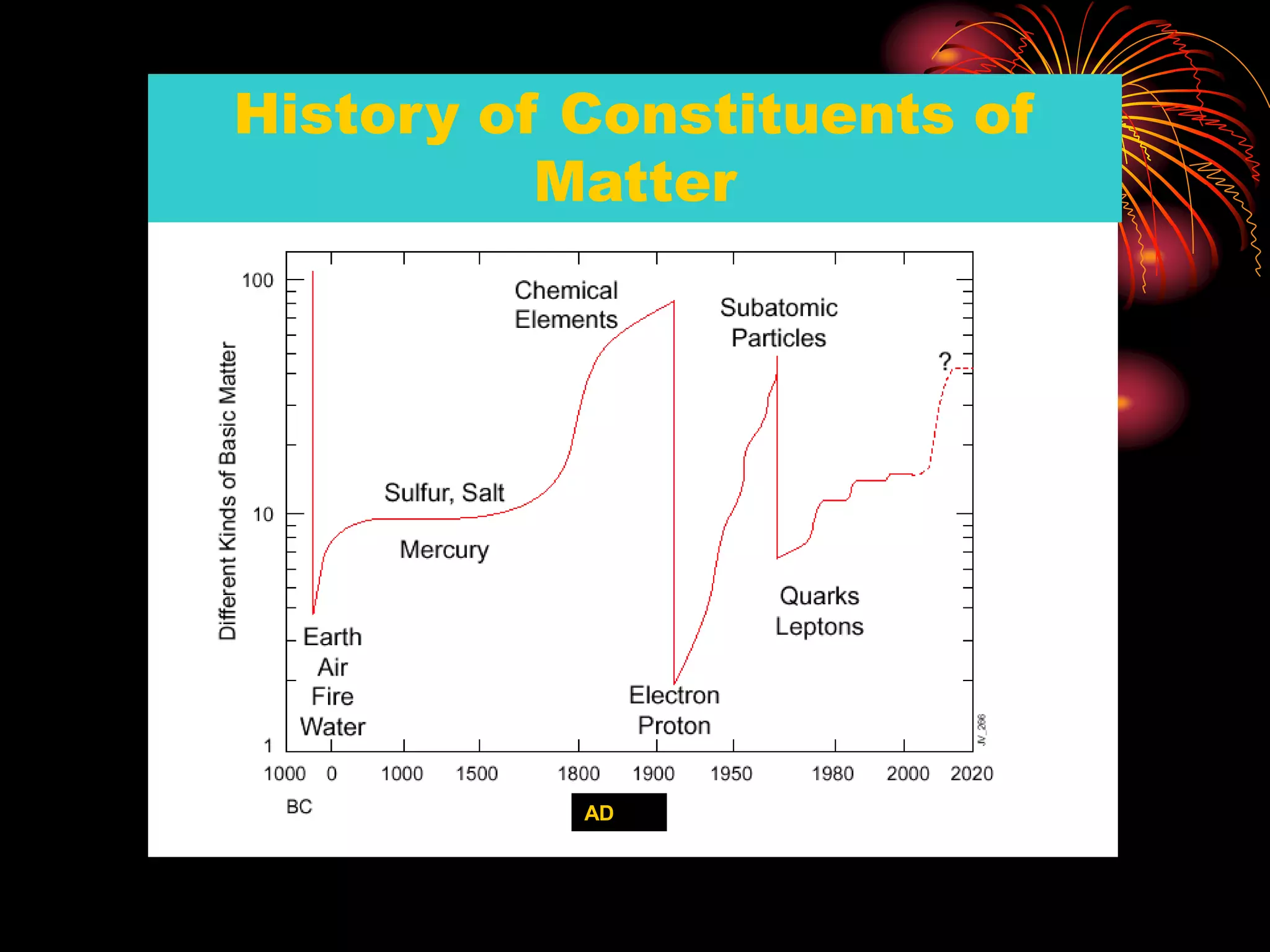
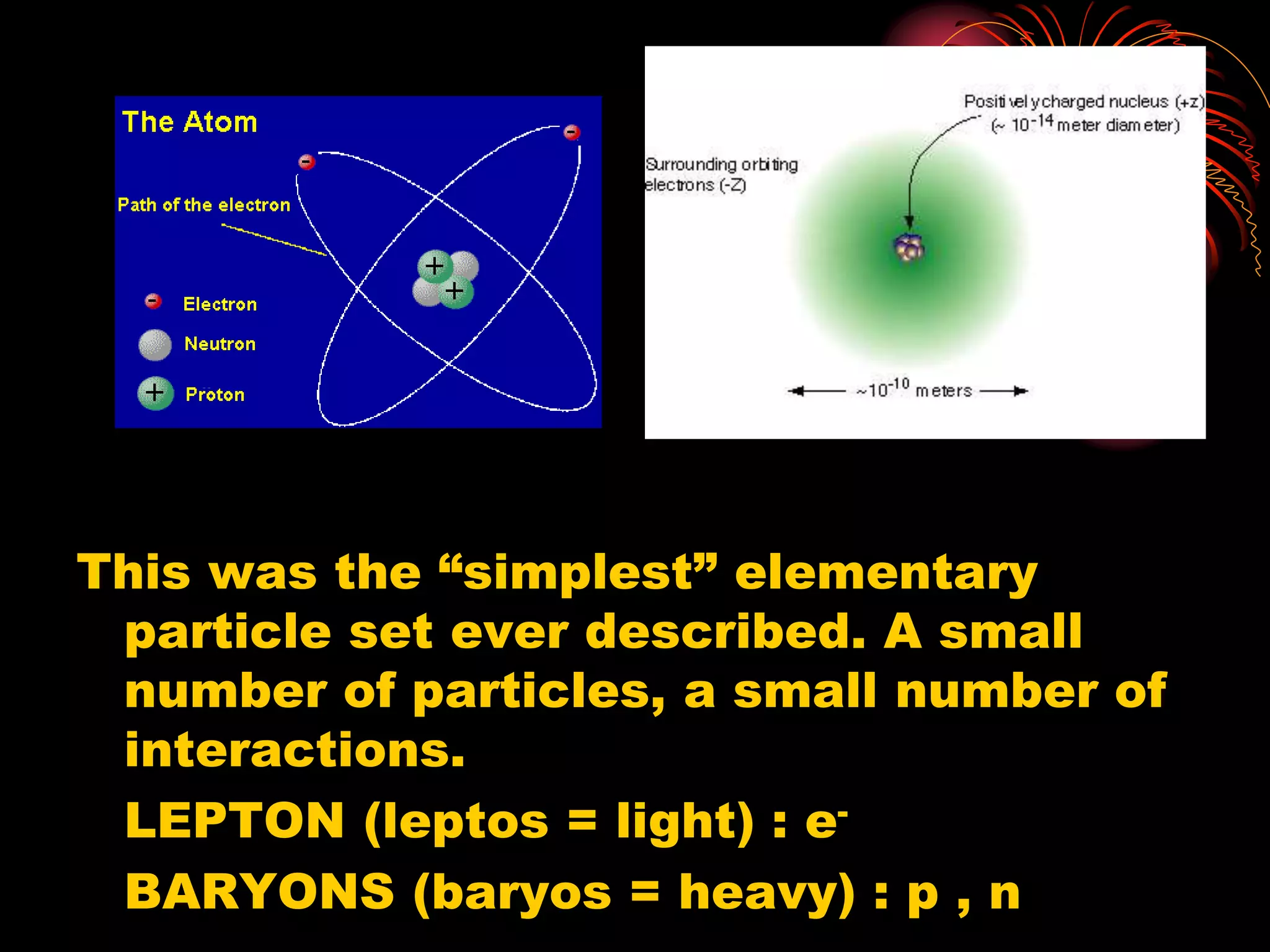
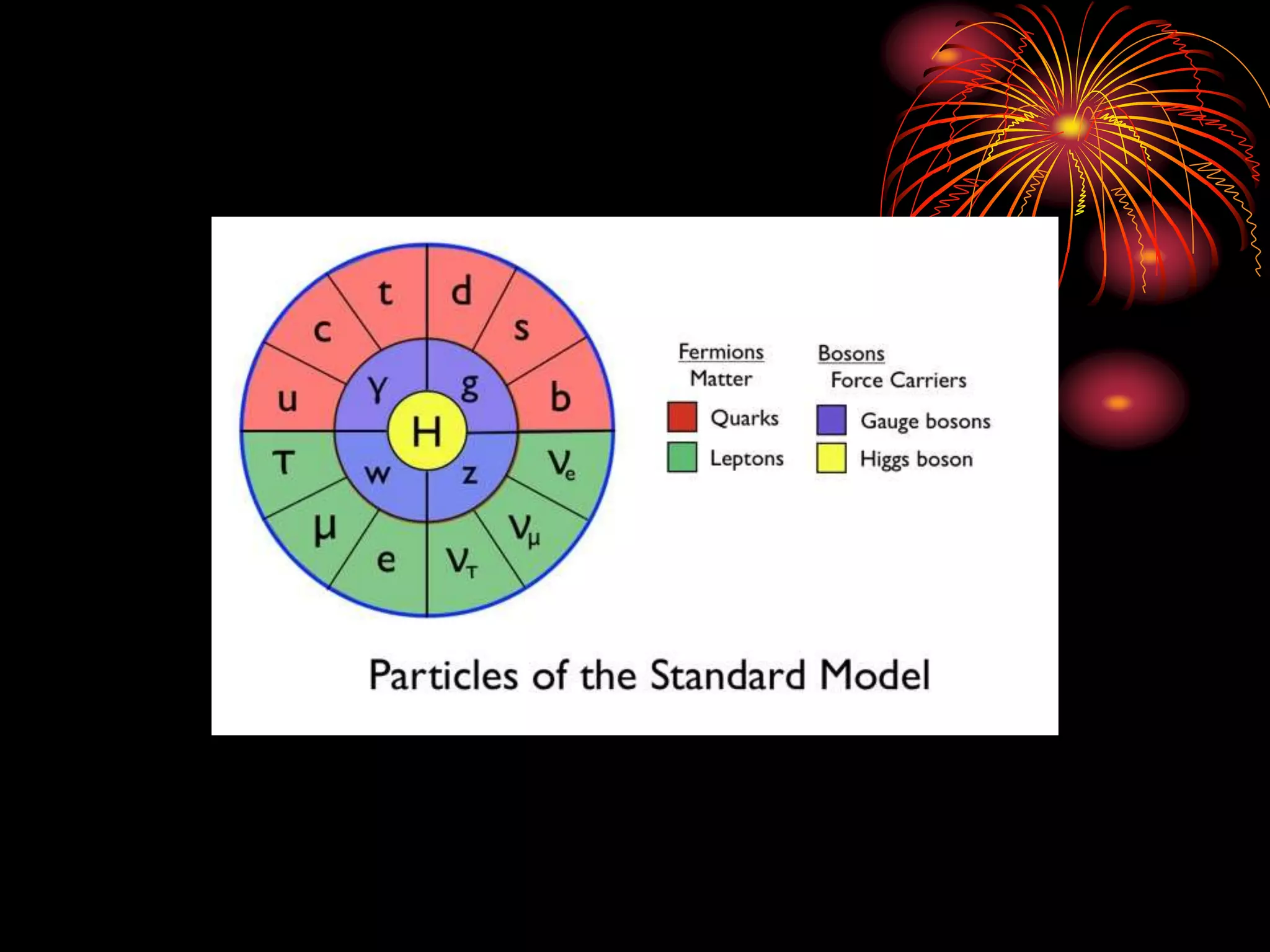
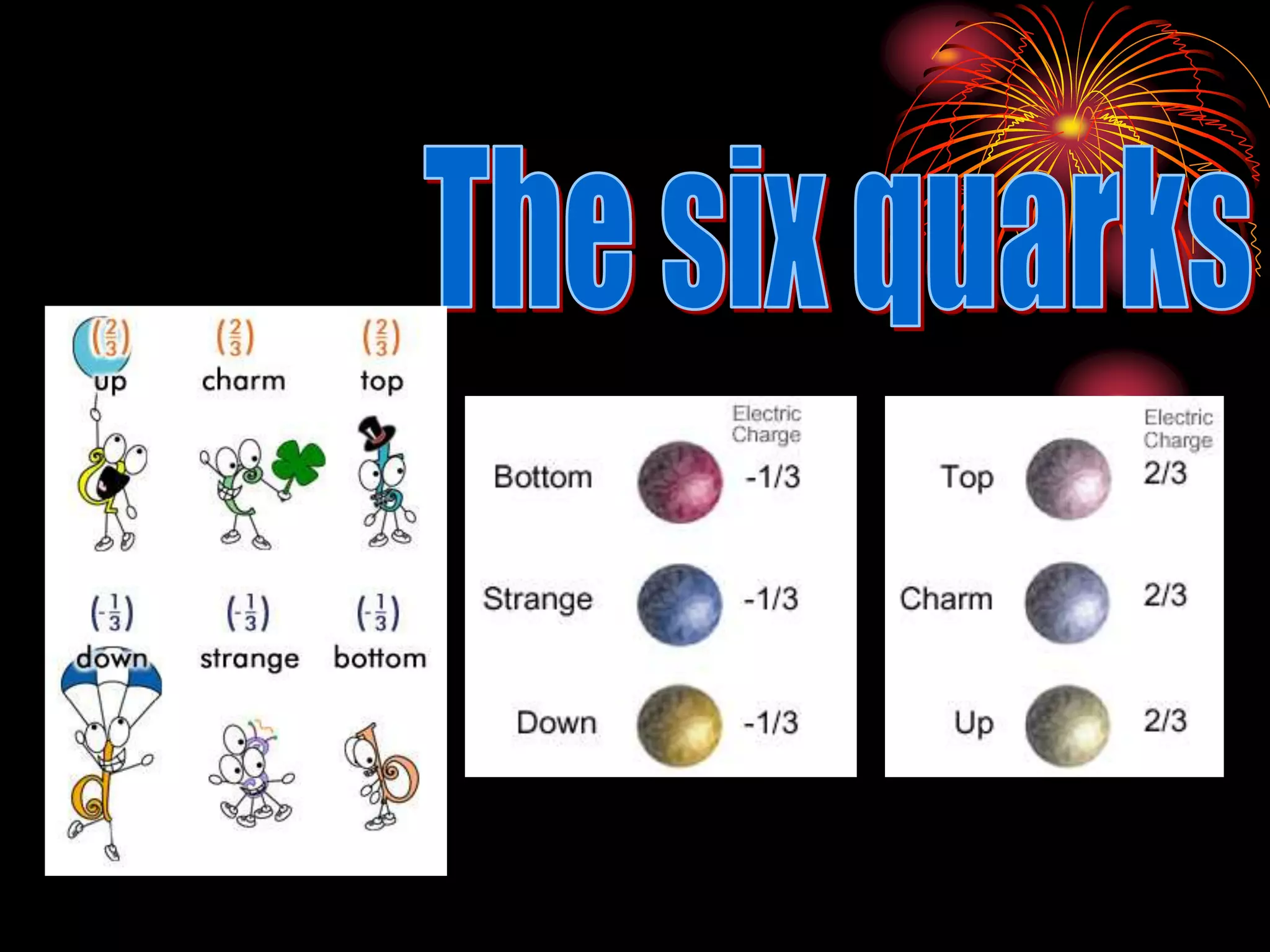
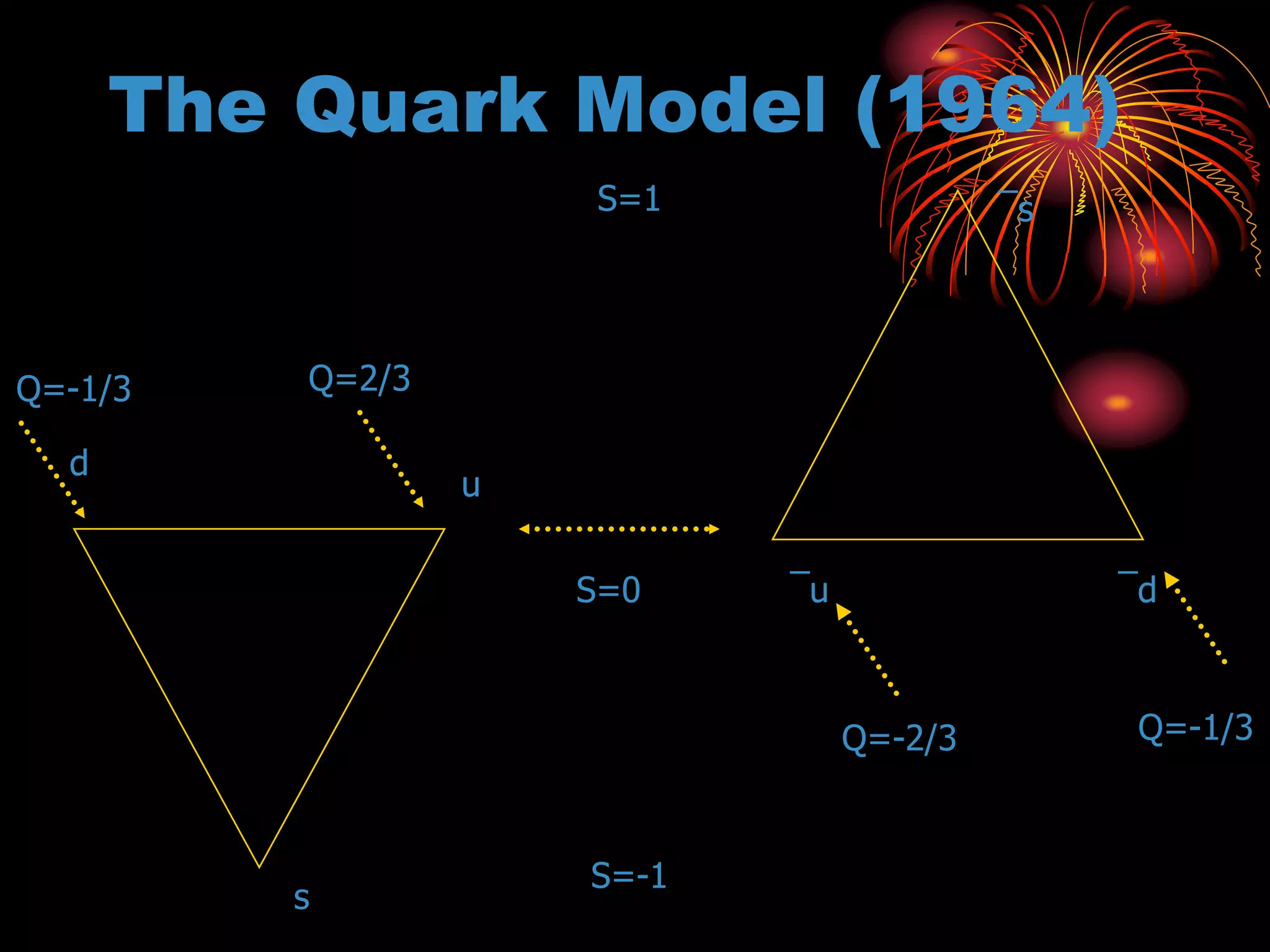
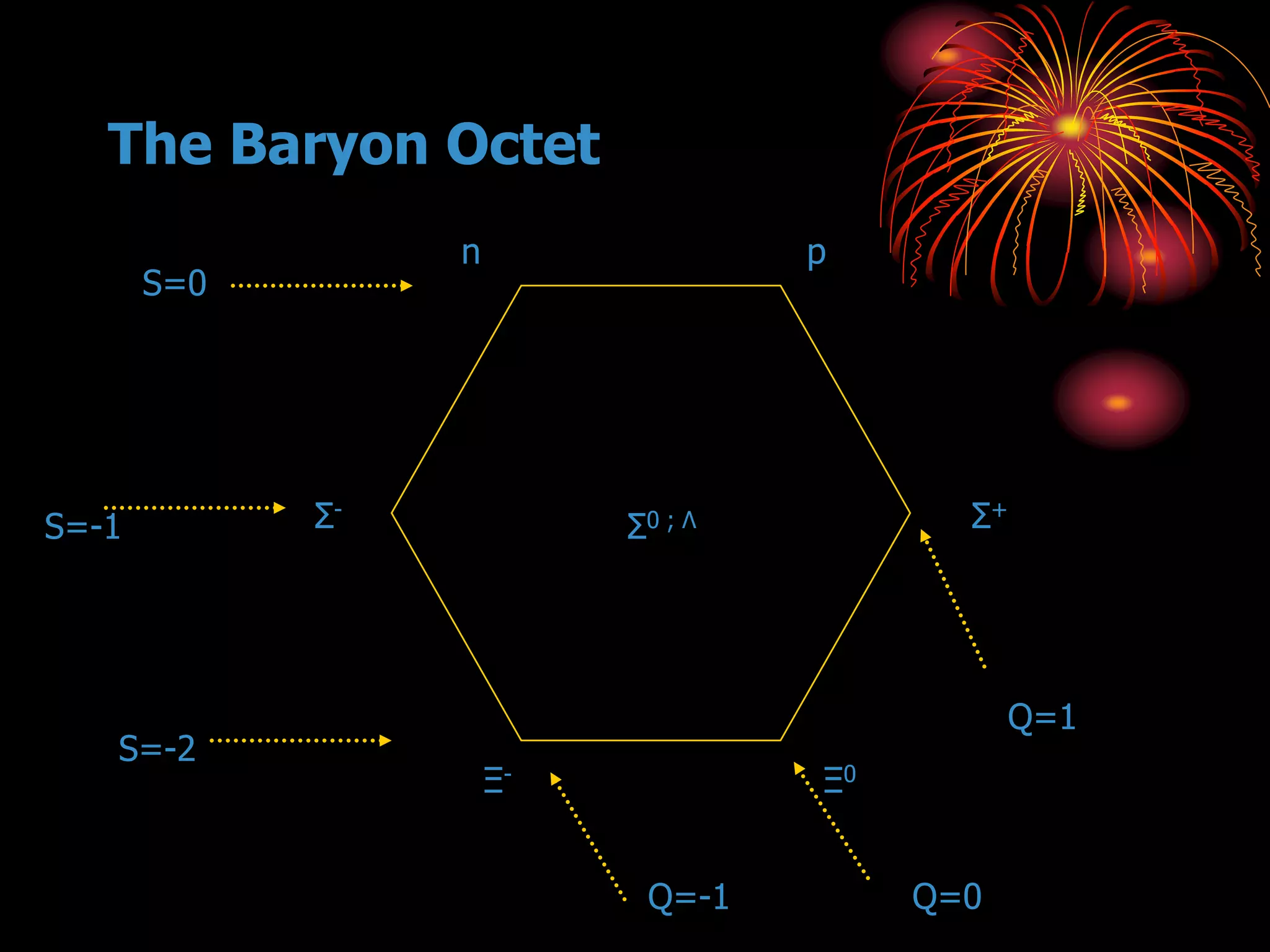
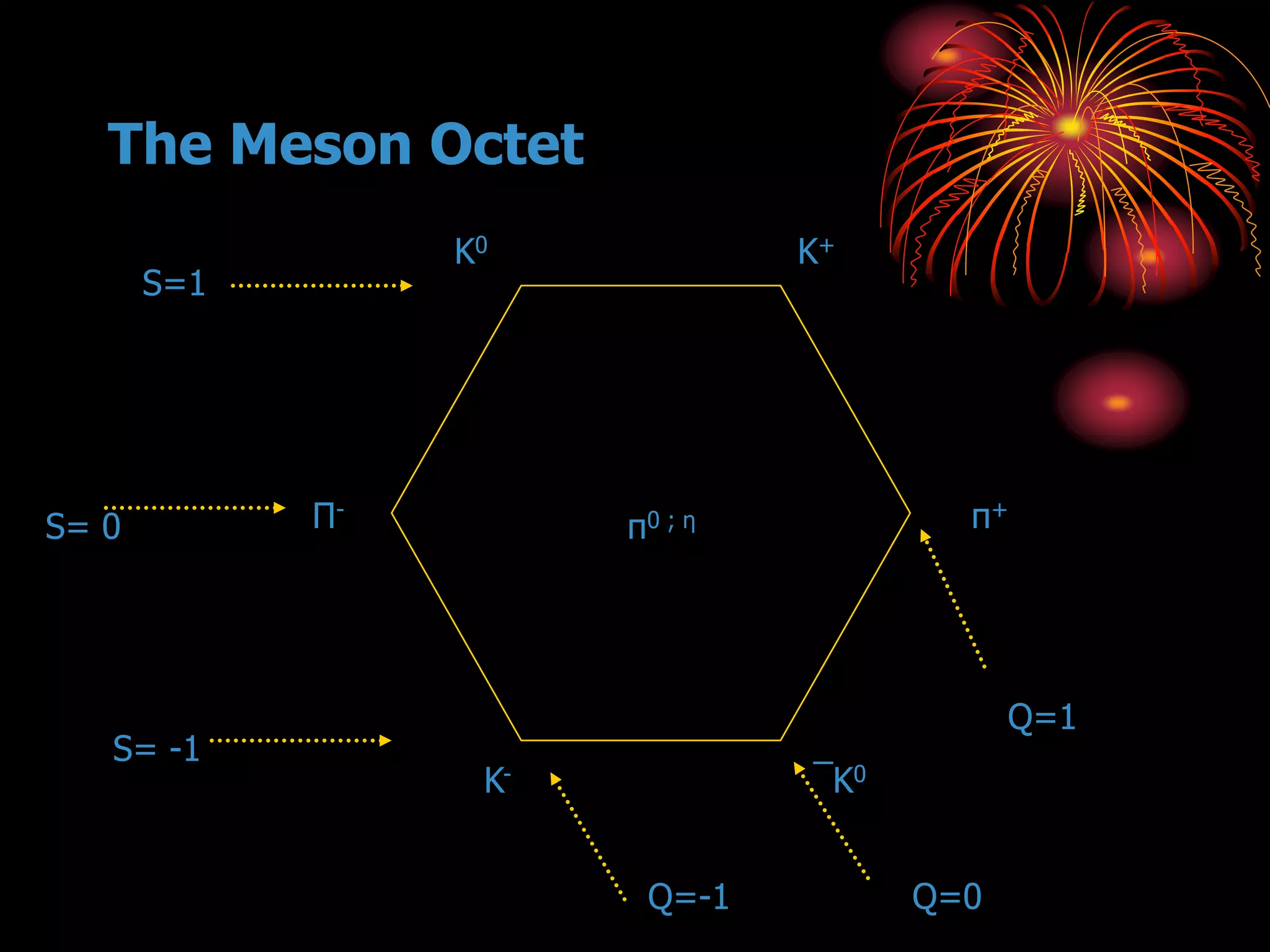
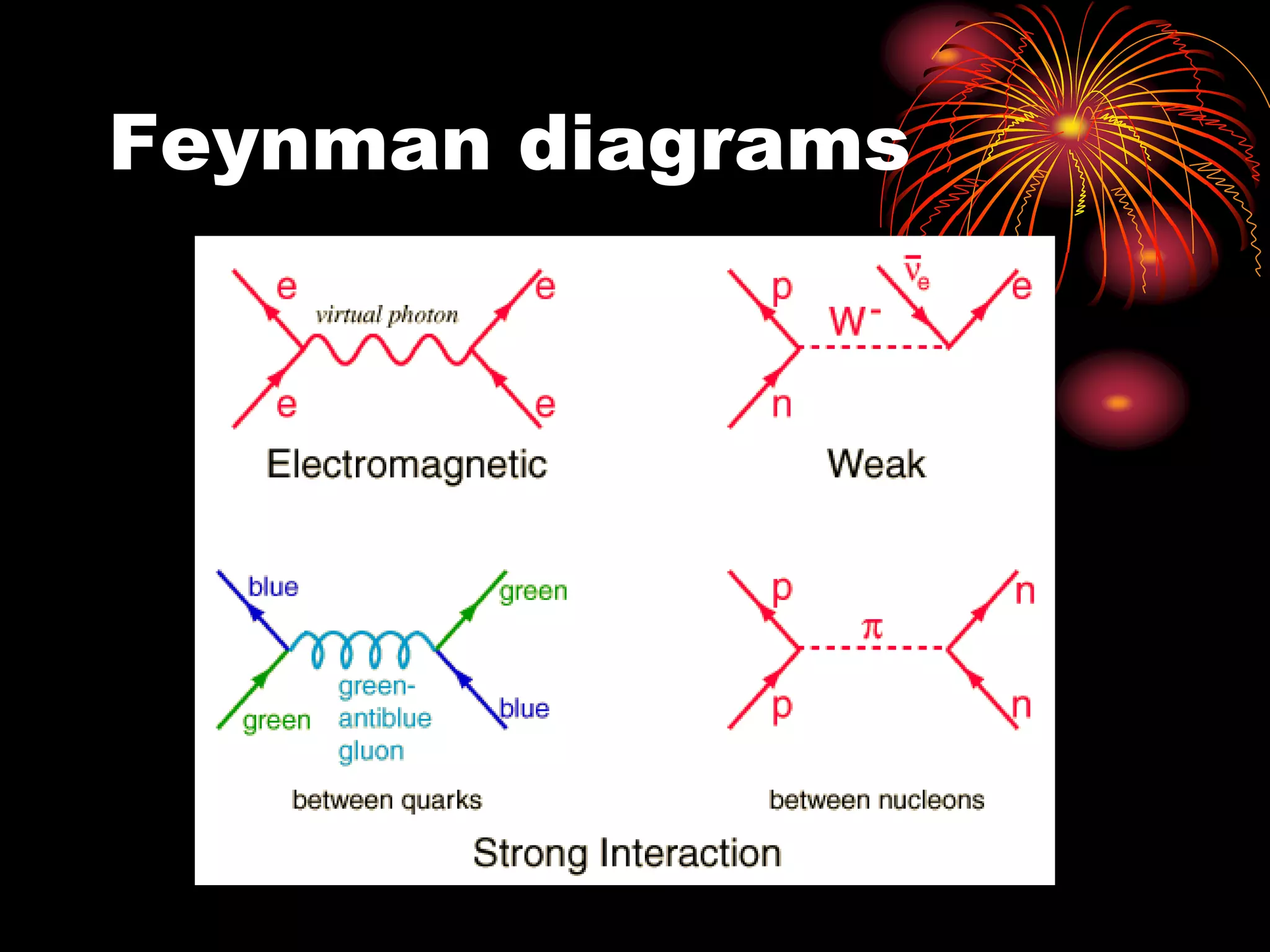
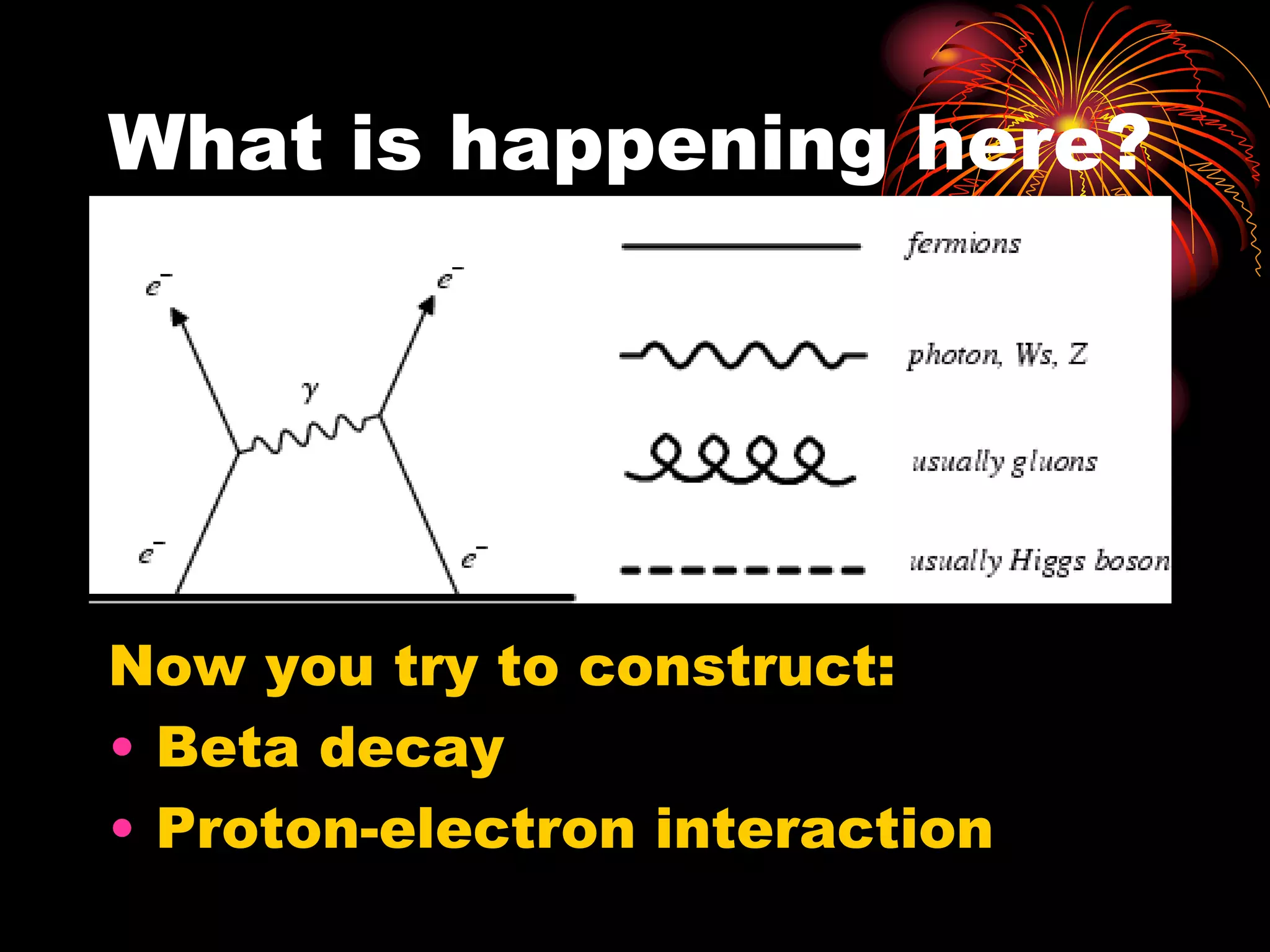
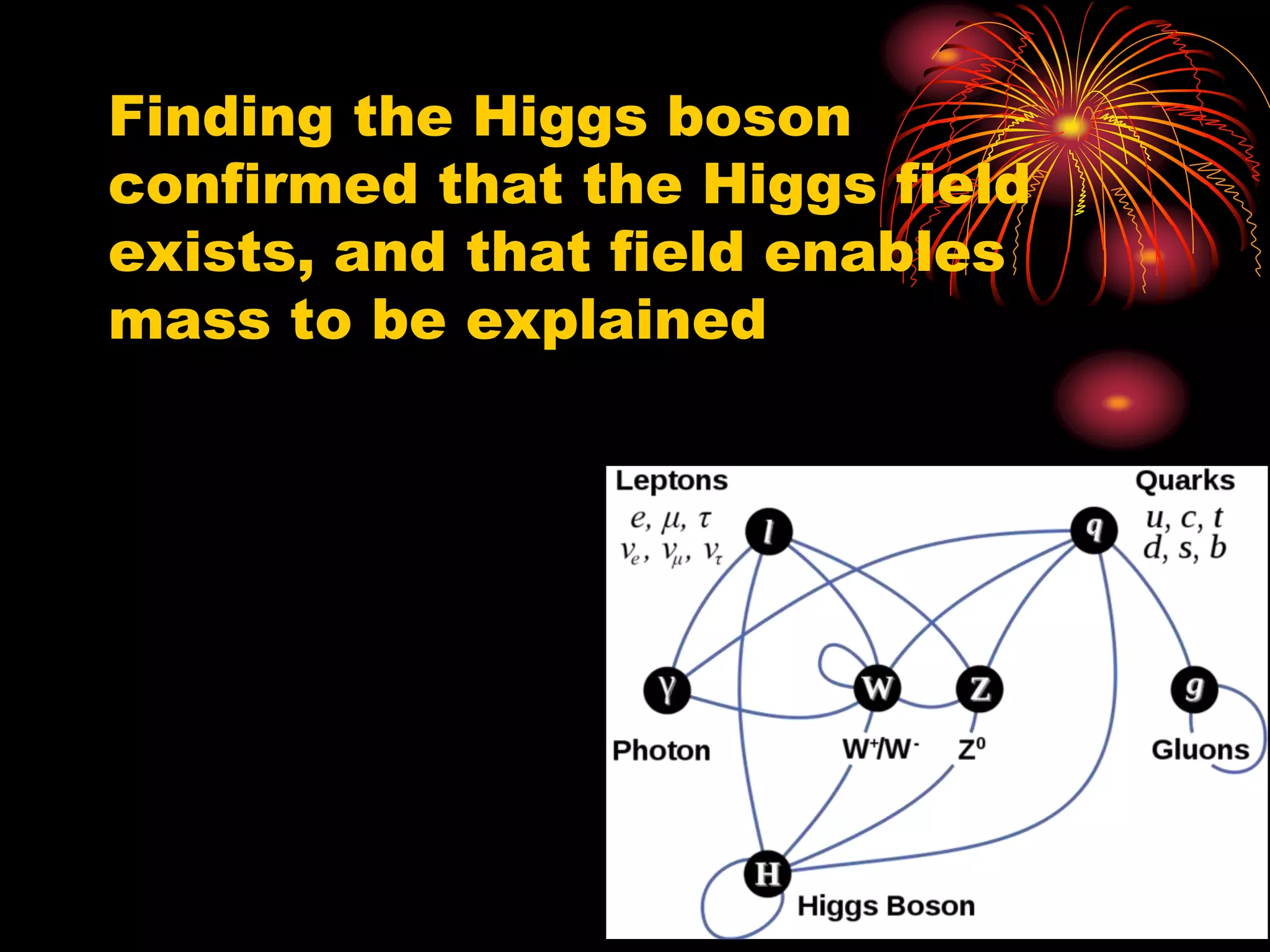
Ernst Rutherford conducted an experiment where he fired alpha particles at a thin gold foil. Most passed straight through but some were scattered back at wide angles. This was unexpected and led Rutherford to propose that atoms have a small, dense nucleus containing positive charge. Later, models showed this could explain the scattering if alpha particles passed close enough to the nucleus to be strongly repelled by its positive charge. This nuclear model revolutionized understanding of atomic structure.