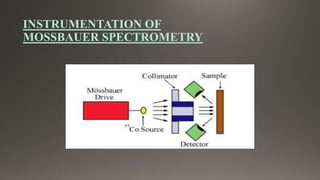
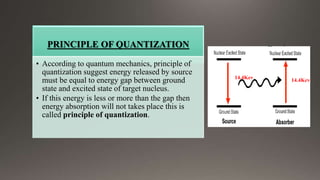
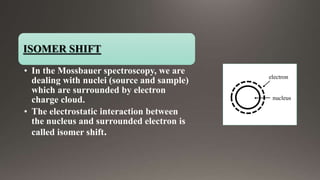
The document provides an overview of Mossbauer spectroscopy, including its history, principles, instrumentation, and various applications in electronic and molecular structure studies. It discusses key concepts such as resonance absorption, recoil motion, Doppler effect, isomer shift, and quadrupole splitting. Additionally, it highlights the significance of Mossbauer spectroscopy in understanding biological functions and outlines various references for further reading.

















![[Mn(H2O)6]𝟐+
• ON THE BASIS OF GEOMETRIES OF COMPLEXES
• ON THE BASIS OF ELECTRONIC CONFIGURATION
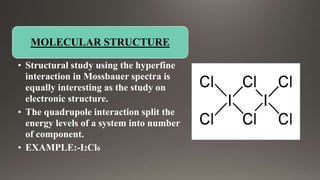
EXAMPLES OF QUADRUPOLE SPLITTING](https://image.slidesharecdn.com/mossbauerspectroscopy-230509035100-a54f31a8/85/mossbauer-spectroscopy-pptx-21-320.jpg)