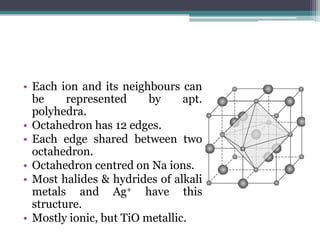
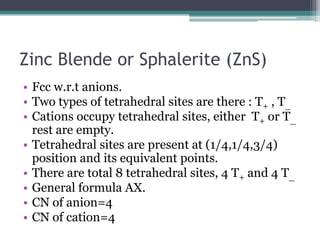
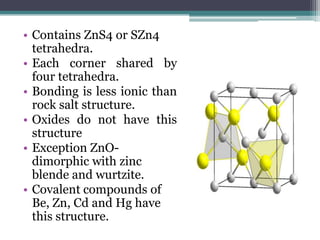
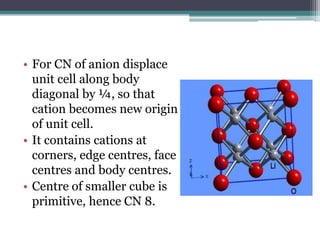
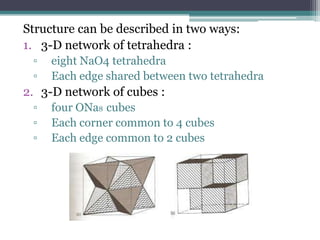
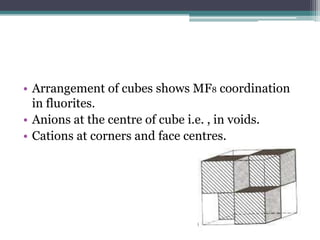
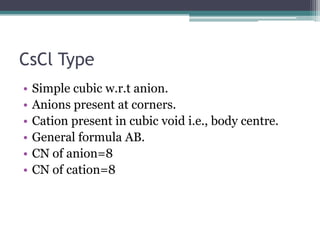
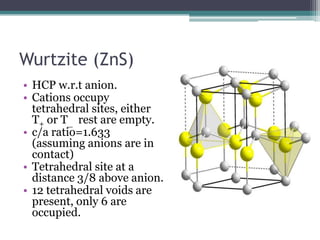
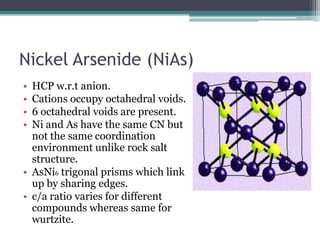
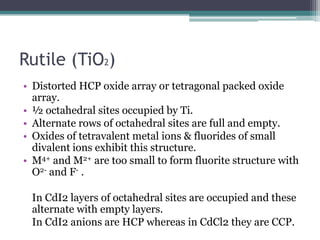
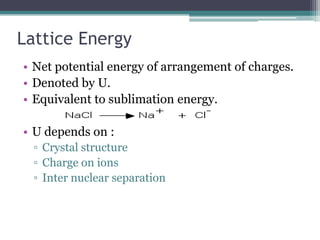
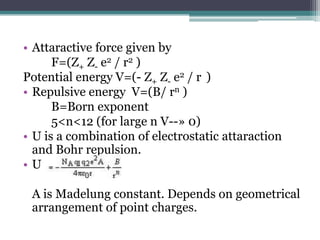
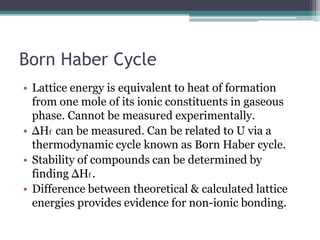
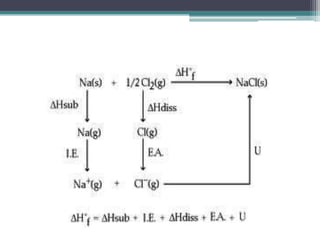
The document describes various ionic structures and their properties, specifically focusing on different types of crystal lattices such as rock salt, zinc blende, antifluorite, fluorite, and others. It highlights the coordination numbers, types of ions occupying certain sites, and the relationship between crystal structure and lattice energy, including the Born-Haber cycle. Additionally, it covers the significance of silicate structures and how charge interactions affect stability and energy in ionic compounds.