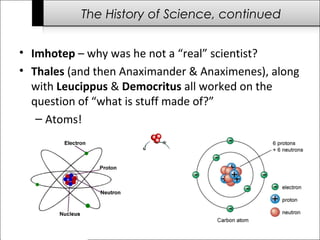
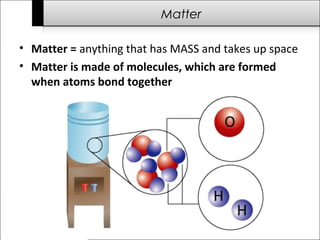
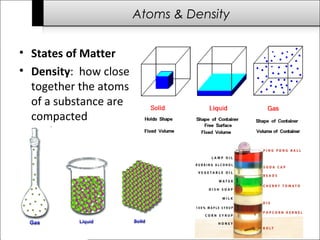
This document provides an overview of the history of science from ancient times to the modern era. It discusses important early scientists and their discoveries, including Imhotep in ancient Egypt, Thales who questioned what matter is made of, and Antoni van Leeuwenhoek who discovered bacteria using microscopes. Later scientists discussed include Isaac Newton, Charles Darwin, Albert Einstein, and James Watson and Francis Crick who discovered the structure of DNA. The document also briefly outlines challenges to science throughout history from groups like the Roman Catholic Church and during the Dark Ages when communication and transportation difficulties slowed scientific progress.