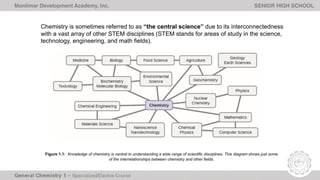
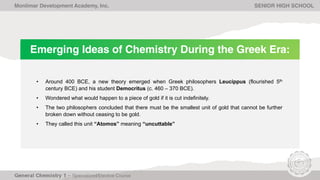
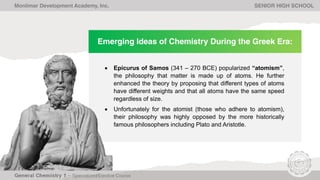
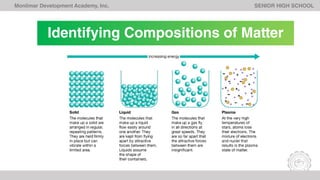
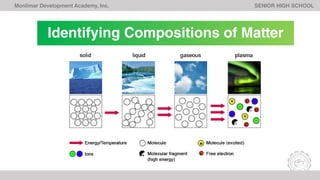
The document outlines Lesson 1 of a General Chemistry course which explores the field and historical development of chemistry, defining what chemistry is, important figures and their contributions, emerging ideas about the nature of matter from ancient Greek philosophers, and the timeline of chemistry's development as a science from prehistory to modern atomic theory. The lesson also discusses the four basic states of matter and compositions of matter, with examples and questions for students.