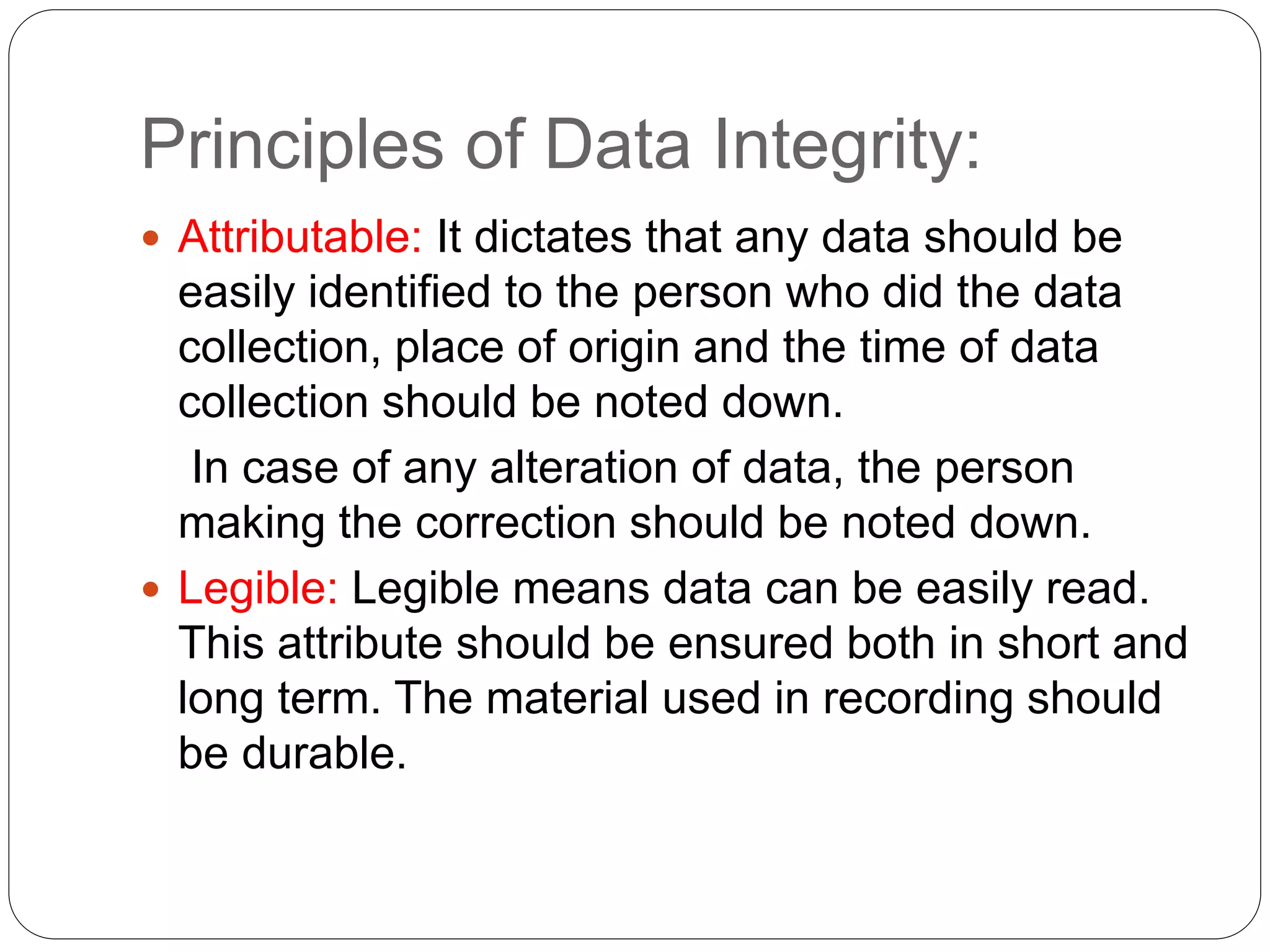
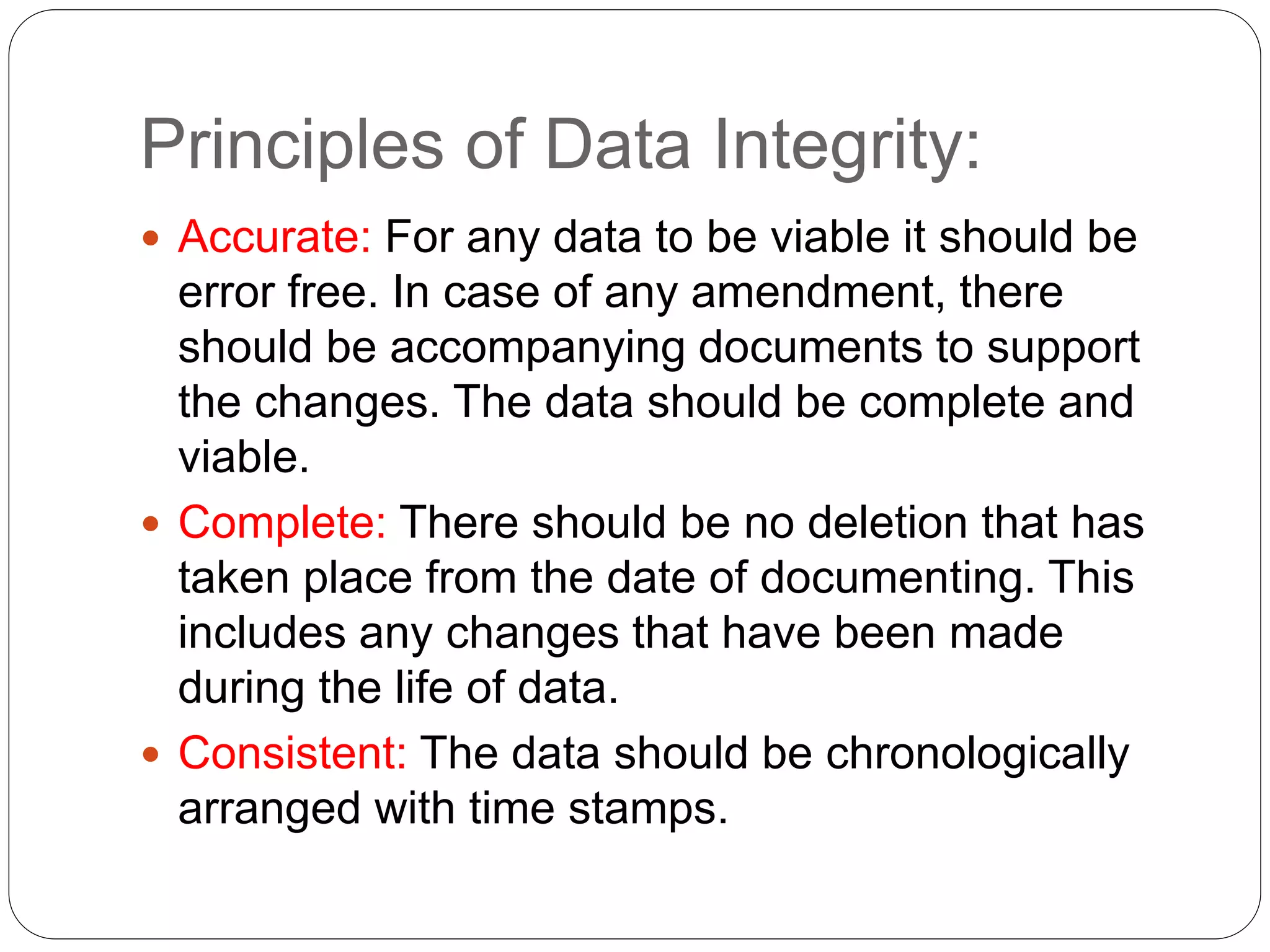
Data integrity ensures that data generated during pharmaceutical business operations and manufacturing is accurate, complete, and reliable to ensure patient safety. It requires that data be attributable, legible, contemporaneous, original, and accurate (ALCOA) - meaning data must be linked to its source, clearly readable, recorded at the time of the relevant activities, reflect original observations, and be correct. Metadata, or data about data, provides necessary context for understanding data values and maintaining data integrity throughout the data lifecycle and regulatory record retention periods. Violations of data integrity can jeopardize patient safety, so it is critical the pharmaceutical industry adhere to data integrity standards and regulatory requirements.