The document discusses data integrity and provides examples of potential integrity issues. It defines data integrity and discusses its importance. Common areas where integrity issues arise include not reporting failed results, fabricating data, deleting electronic records, and disabling audit trails. Consequences of integrity breaches include regulatory actions, loss of customer trust and business, and employment consequences. The document also provides examples of integrity issues found during audits such as missing documentation, imperfect records, and discrepancies between records and actual observations.



![DATA INTEGRITY
WHAT IS INTEGIRY
Is what you are doing,saying,Recording is REAL!!
[in-teg-ri-tee]
1.Adherence to moral and ethical principles;soundness of moral character;
honesty.
2. The state of being whole, entire, or undiminished:
To preserve the integrity of the empire.
3. A sound,unimpaired,or perfect condition:
The integrity of a ship's hull.](https://image.slidesharecdn.com/dataintegrity-230125042742-f869a768/85/Data-Integrity-pptx-4-320.jpg)

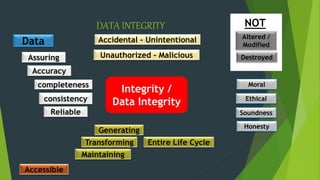
![DATA INTEGRITY
5. Unauthorized manner. "Data integrity refers to maintaining and assuring
the accuracy and consistency of data over its entire life-cycle.
[Wikipedia]
6.Data Integrity is the assurance that data is consistent, accurate, reliable
and accessible](https://image.slidesharecdn.com/dataintegrity-230125042742-f869a768/85/Data-Integrity-pptx-6-320.jpg)