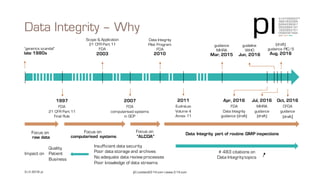
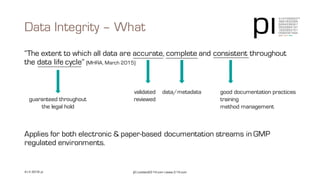
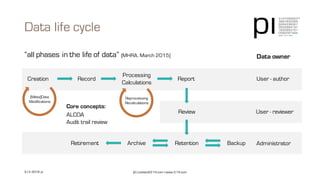
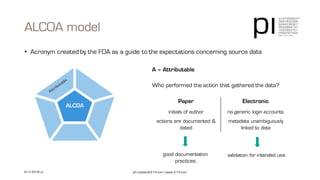
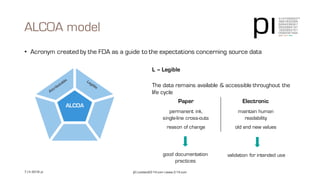
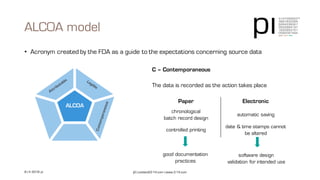
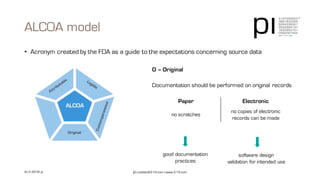
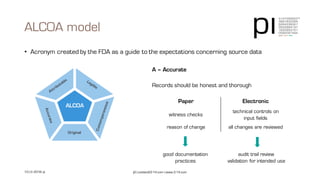
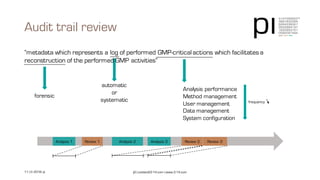
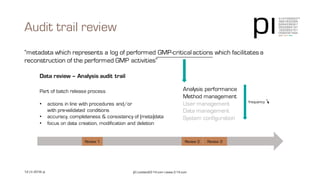
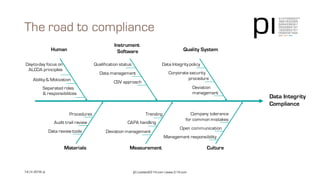
The document outlines the importance of data integrity in regulated environments, highlighting key concepts such as the data life cycle and the ALCOA principles (Attributable, Legible, Contemporaneous, Original, Accurate). It discusses compliance actions, including short and mid-term strategies for improving data integrity in the pharmaceutical industry. The document emphasizes that data integrity directly impacts patient safety, the quality of products, and the efficiency of data flows.