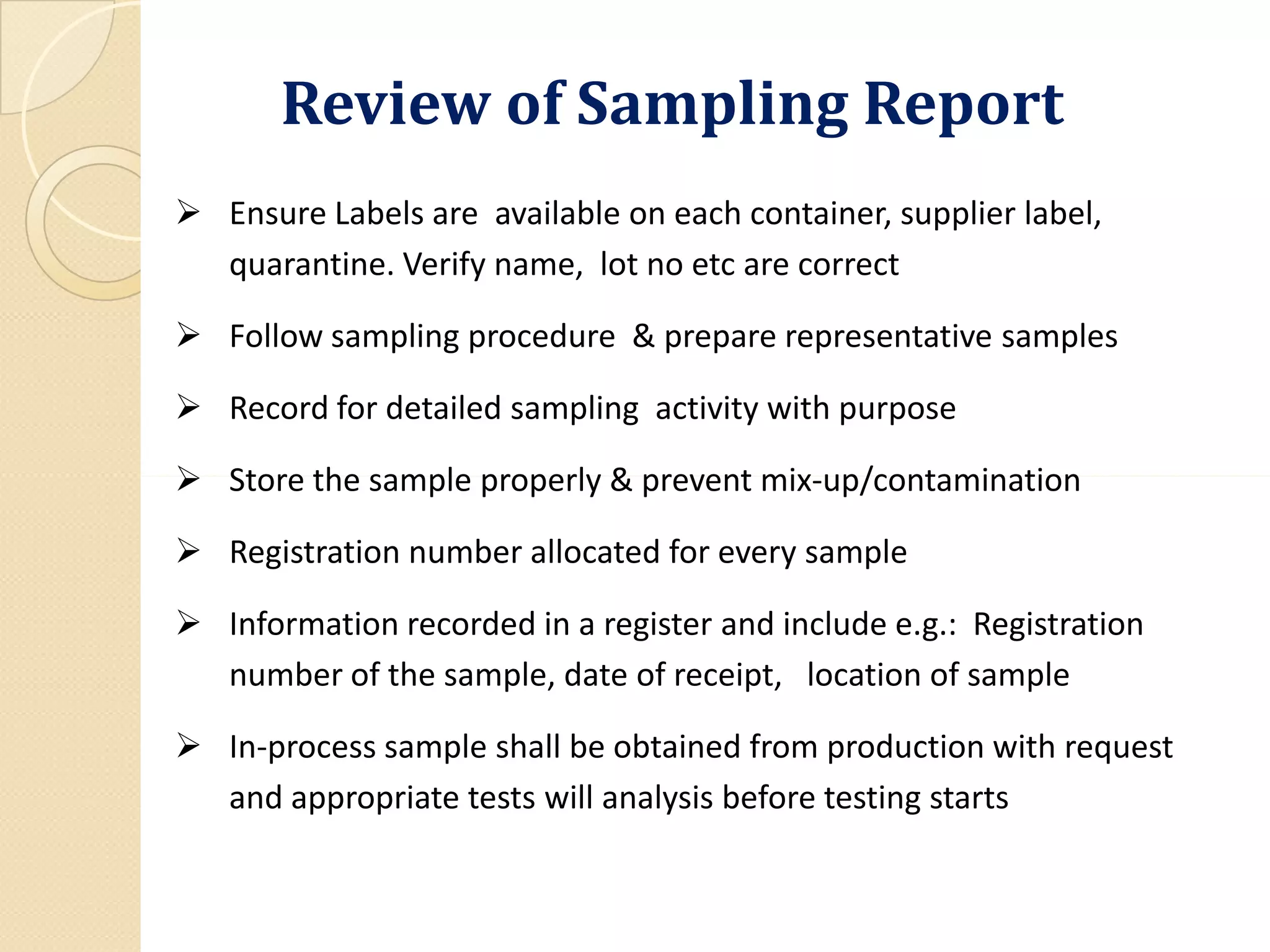
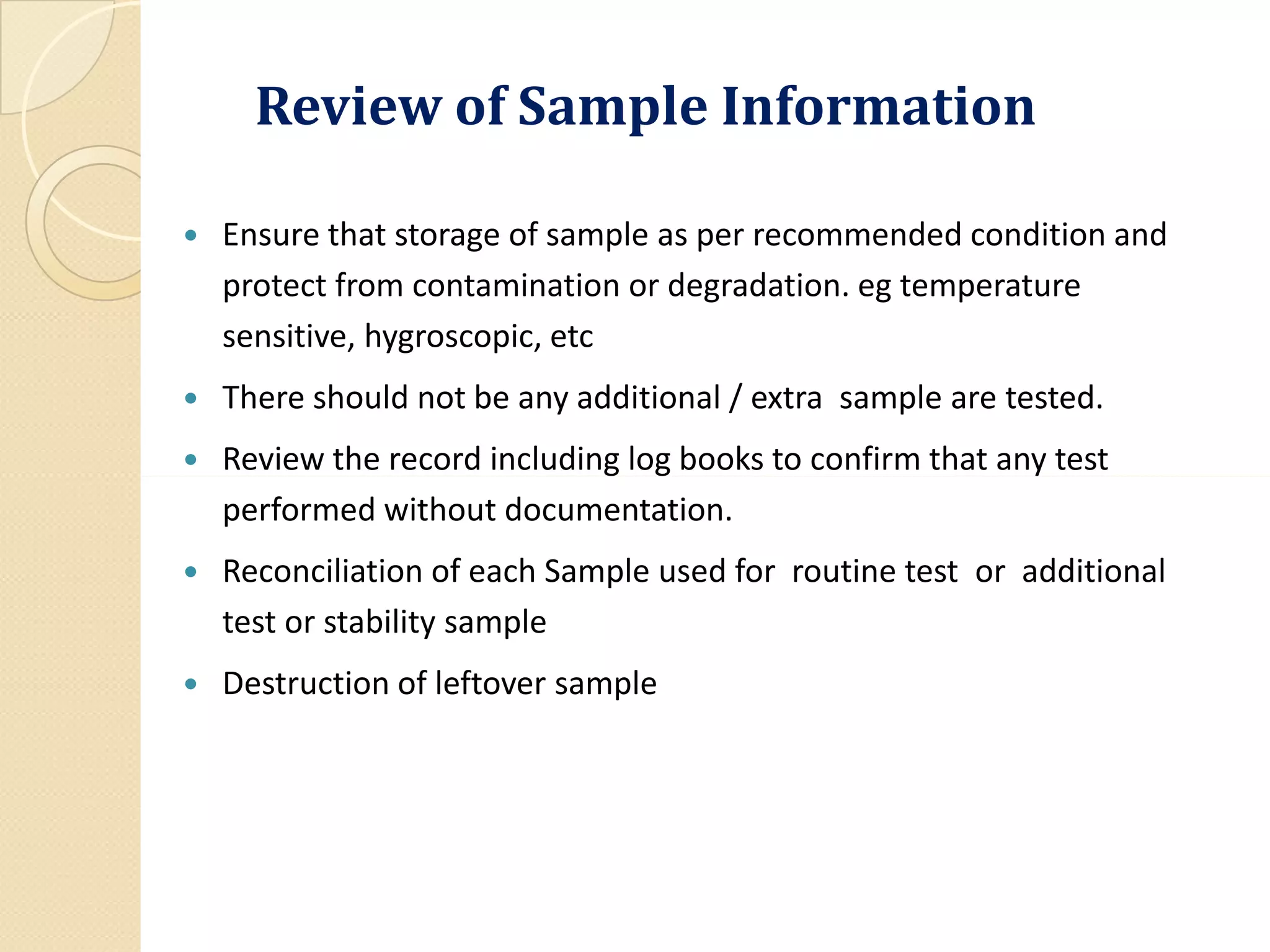
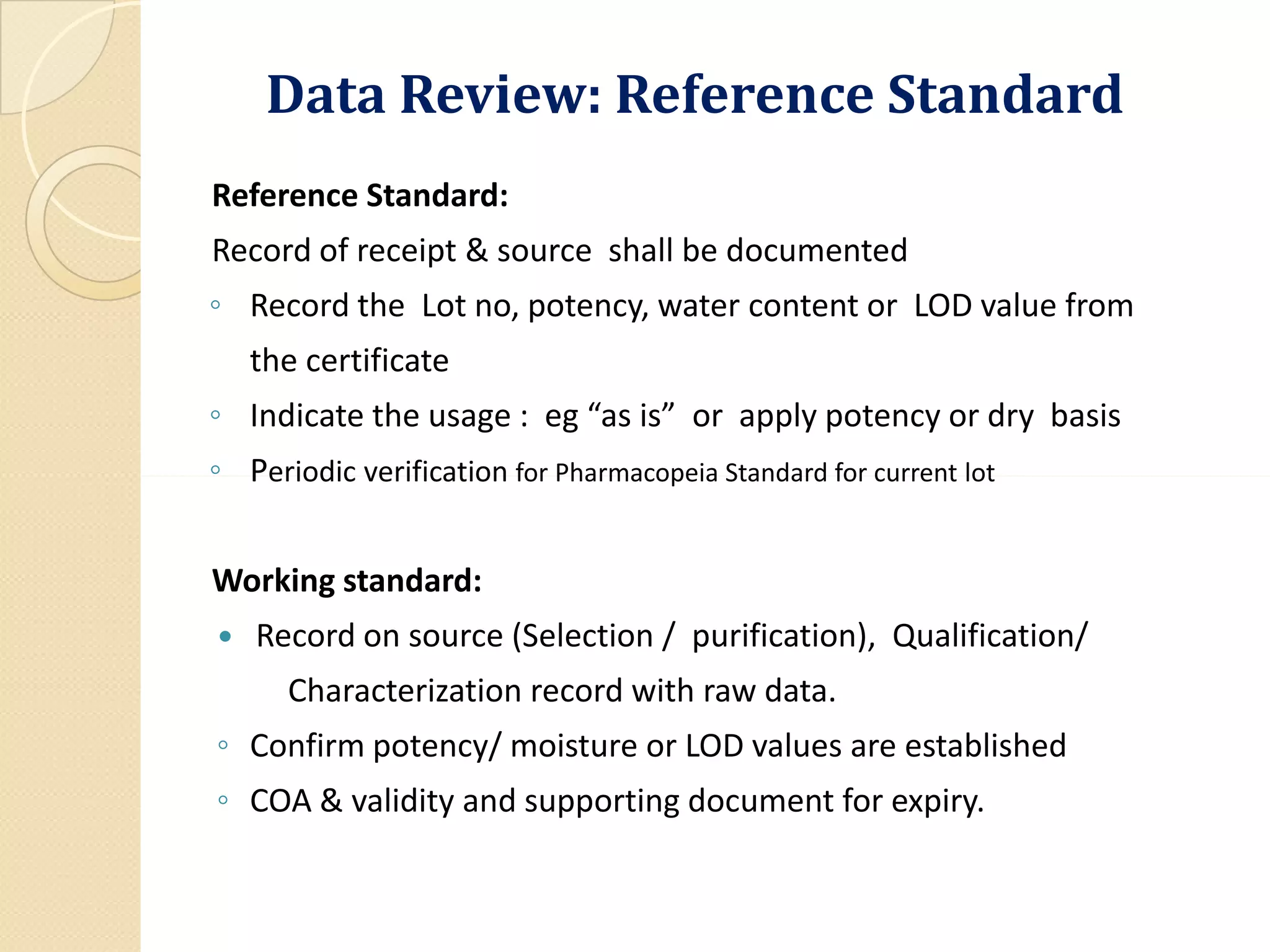
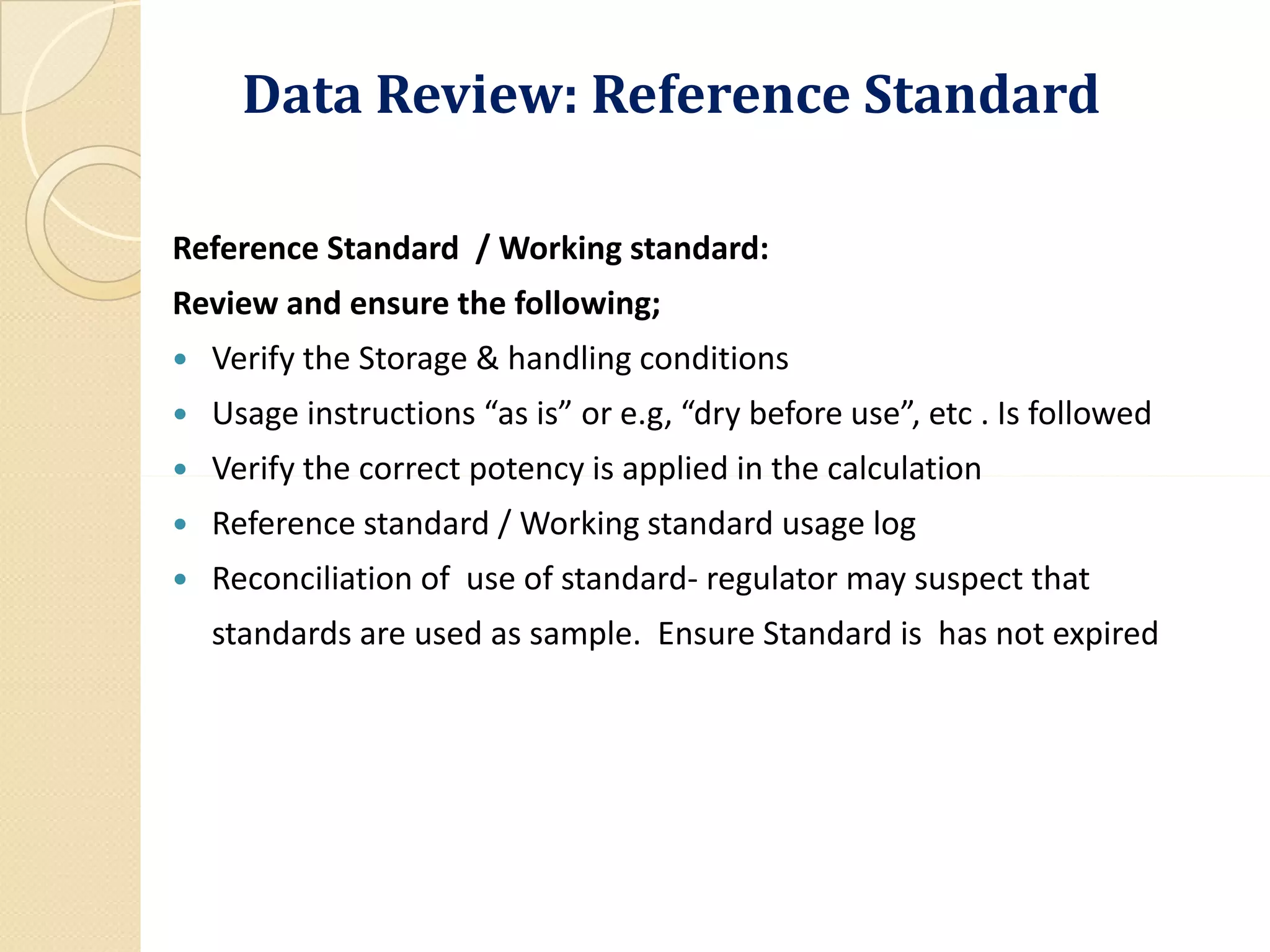
The document outlines the critical importance of reviewing quality control (QC) records and analytical data in compliance with Good Manufacturing Practices (GMP). It emphasizes the need for accuracy and integrity in data to ensure product safety and regulatory compliance, detailing methodologies, procedures, and responsibilities involved in QC reviews. Necessary documentation and audit trails are highlighted, along with various GMP requirements and definitions related to data integrity and quality assurance.






![GMP Requirement :QC Record& ReviewGMP Requirement :QC Record& Review
ICH Q7 “Laboratory Control Records”. 6.60 & 6.61
6.60: Laboratory control records should include complete data derived
from all tests conducted to ensure compliance with established
specifications and standards, including examinations and assays, as
follows:
A description of samples received for testing, including the material A description of samples received for testing, including the material
name or source, batch number or other distinctive code, date sample
was taken, and, where appropriate, the quantity and date the sample
was received for testing; [ 211.194 (a)1]
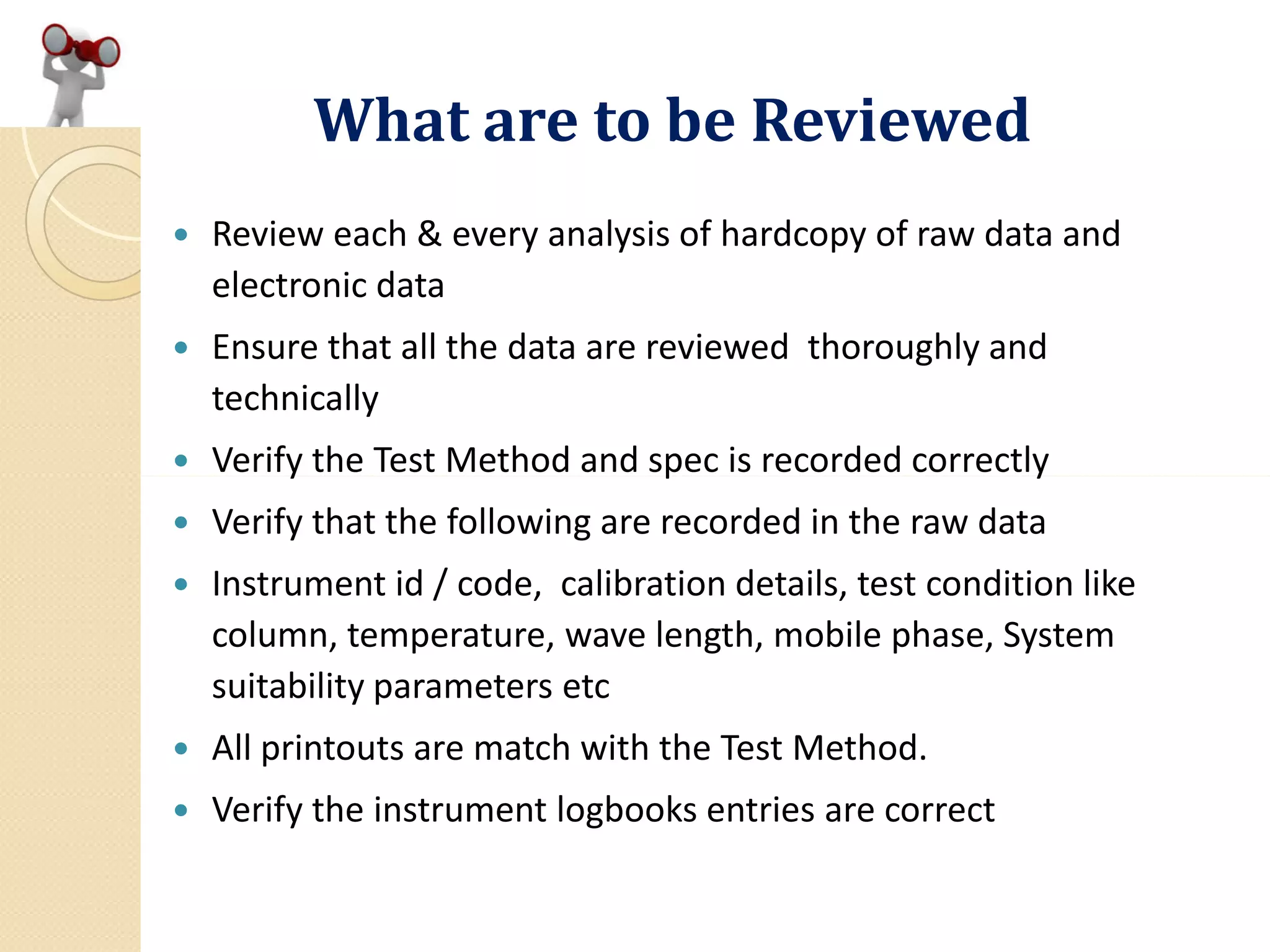
A statement of or reference to each test method used; [ 211.194 (a)2]
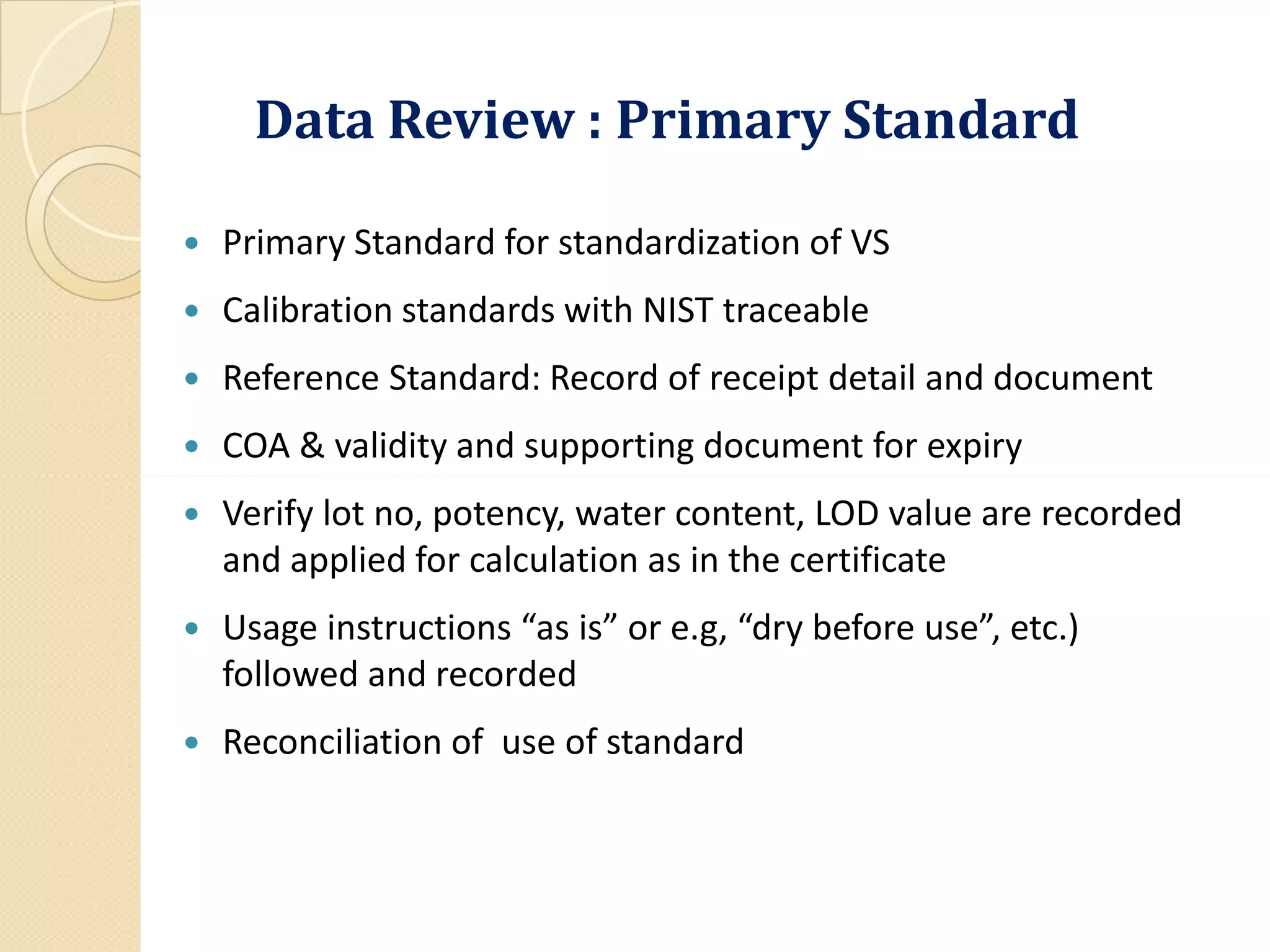
Use Standard is USP-NF).
A statement of the weight or measure of sample used for each test as
described by the method; data on or cross-reference to the
preparation and testing of reference standards, reagents and standard
solutions; [ 211.194 (a)3]](https://image.slidesharecdn.com/reviewofqualitycontrolrecordandanalyticaldatadr-200321164851/75/Review-of-Quality-Control-Record-and-Analytical-Data-by-Dr-A-Amsavel-11-2048.jpg)
![GMP Requirement :QC Record& ReviewGMP Requirement :QC Record& Review
ICH Q7 “Laboratory Control Records”. 6.60
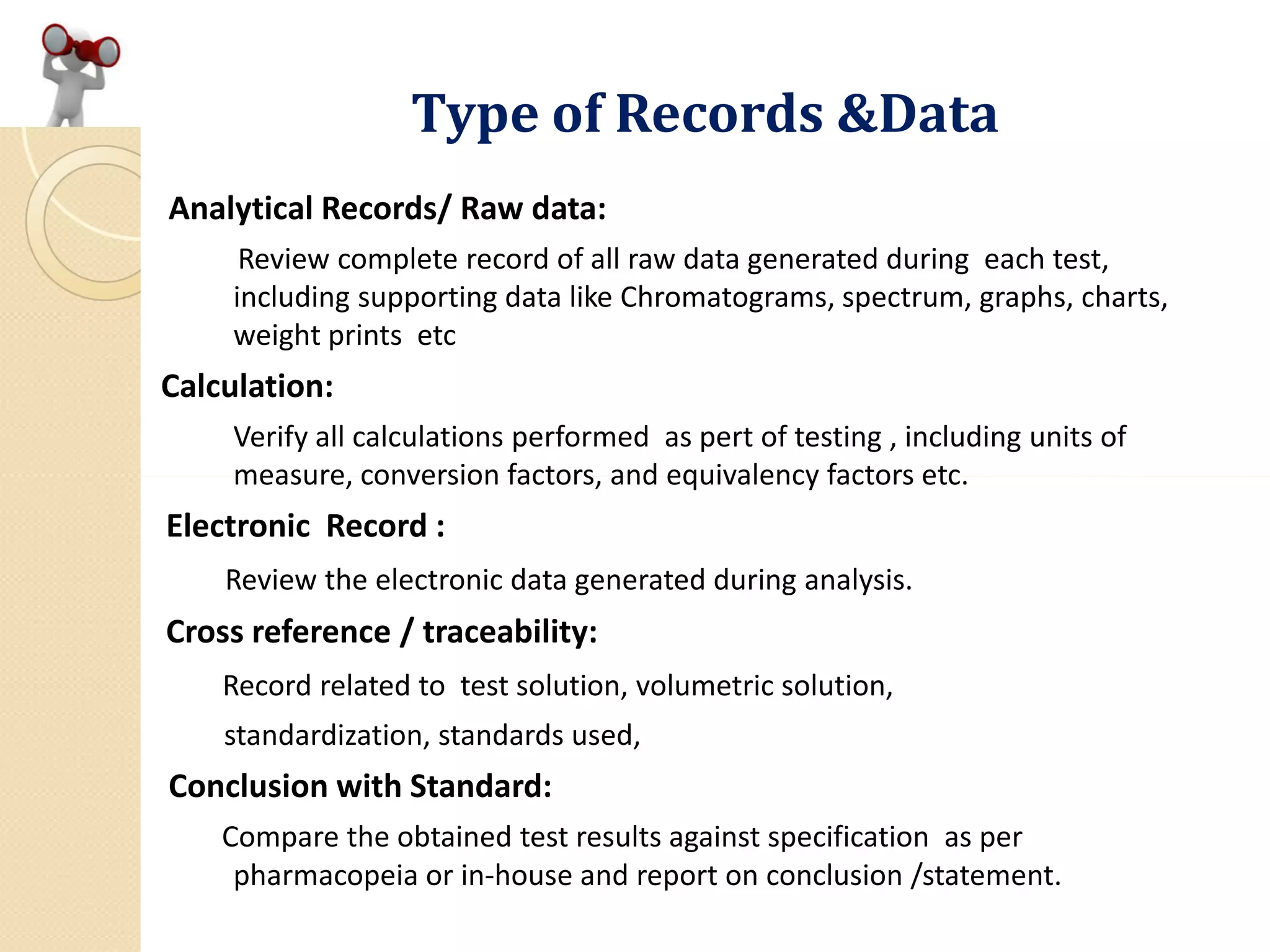
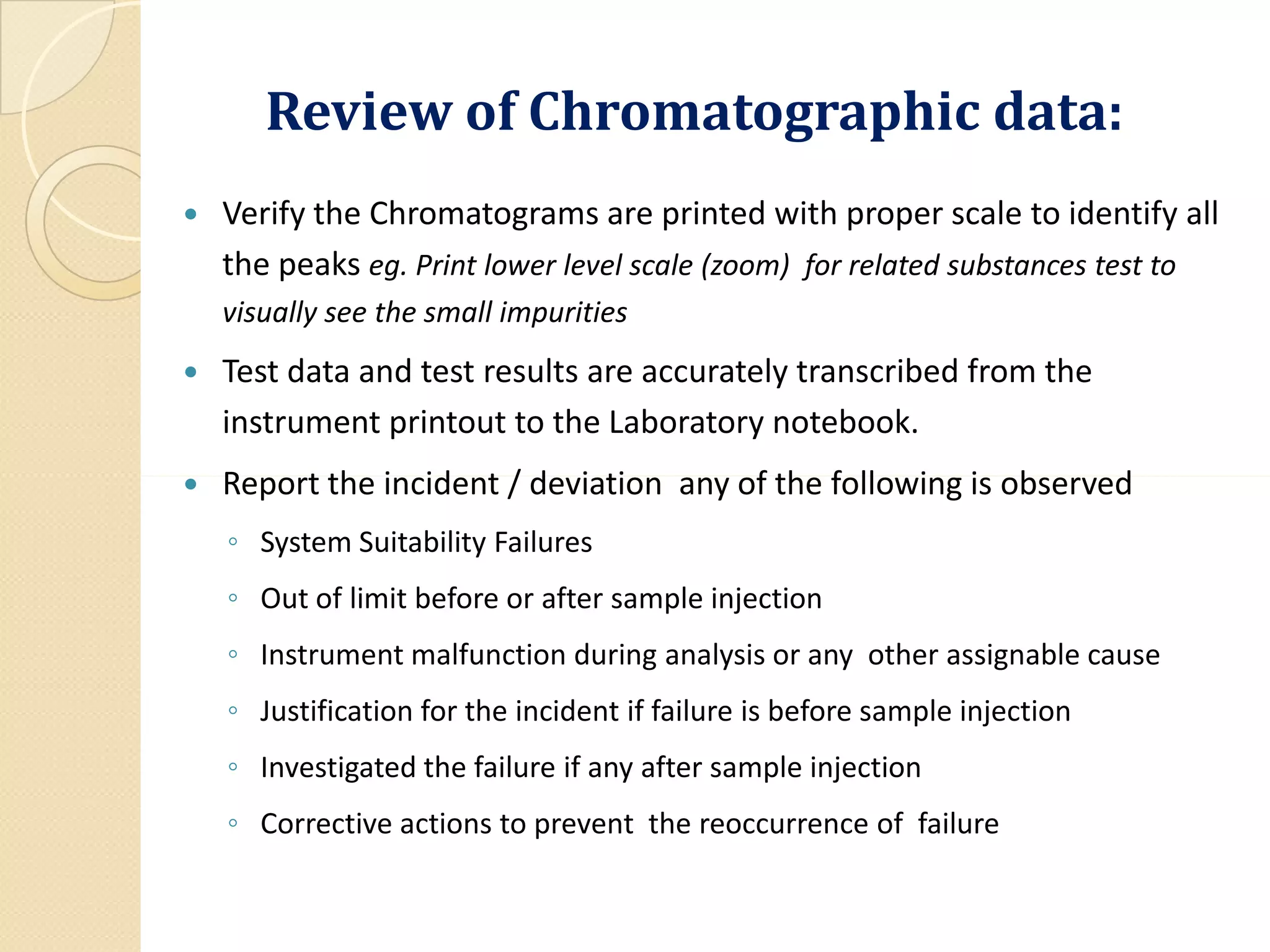
A complete record of all raw data generated during each test,
in addition to graphs, charts, and spectra from laboratory
instrumentation, properly identified to show the specific
material and batch tested; [211.194 (a)4]
A record of all calculations performed in connection with the
test, including, for example, units of measure, conversion
factors, and equivalency factors; [211.194 (a)5]
A statement of the test results and how they compare with
established acceptance criteria; [211.194 (a)6]
The signature of the person who performed each test and
the date(s) the tests were performed; [211.194 (a)7]](https://image.slidesharecdn.com/reviewofqualitycontrolrecordandanalyticaldatadr-200321164851/75/Review-of-Quality-Control-Record-and-Analytical-Data-by-Dr-A-Amsavel-12-2048.jpg)
![GMP Requirement :QC Record& ReviewGMP Requirement :QC Record& Review
ICH Q7 “Laboratory Control Records”. 6.60 (cont..)
The date and signature of a second person showing that the
original records have been reviewed for accuracy, completeness,
and compliance with established standards. [ 211.194 (a)8].
Laboratory Control records” 6.61Laboratory Control records” 6.61
Complete records should also be maintained for:
Any modifications to an established analytical method; [211.194 (b)]
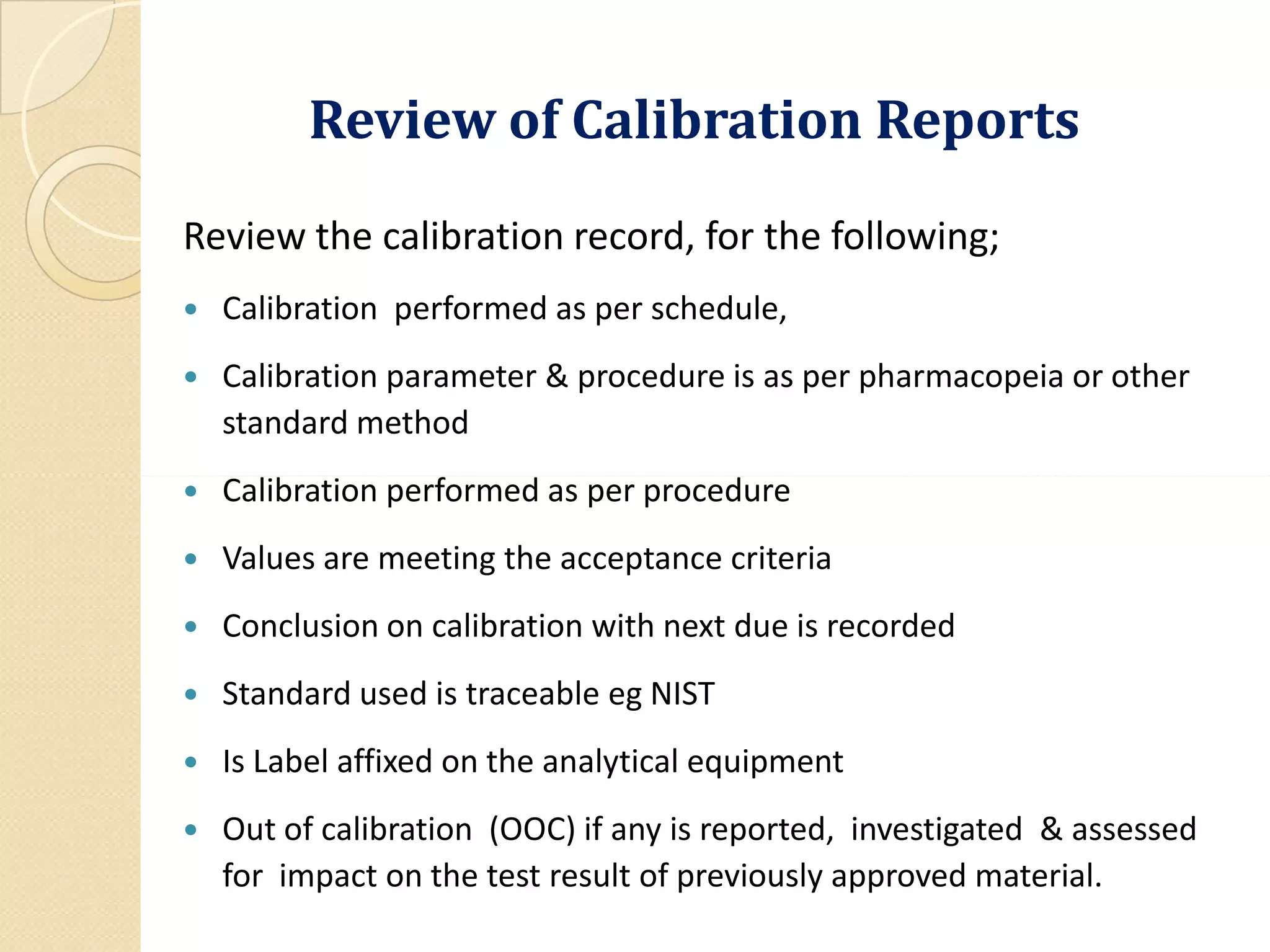
Periodic calibration of laboratory instruments, apparatus, gauges,
and recording devices; [211.194 (d)]
All stability testing performed on APIs; [ 211.194 (e)]
Out-of-specification (OOS) investigations.](https://image.slidesharecdn.com/reviewofqualitycontrolrecordandanalyticaldatadr-200321164851/75/Review-of-Quality-Control-Record-and-Analytical-Data-by-Dr-A-Amsavel-13-2048.jpg)