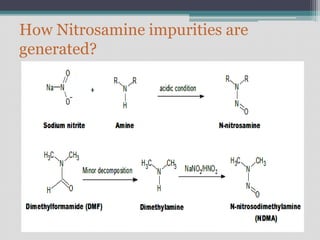
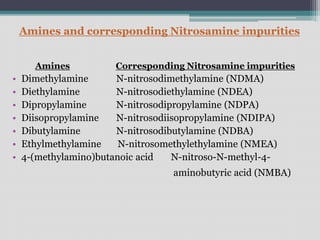
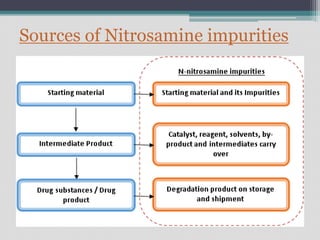
The document discusses nitrosamine impurities, which are carcinogenic compounds that have been found in some generic drug substances and products. It notes that regulatory agencies announced the presence of specific nitrosamines like NDMA and NDEA in drugs like ARBs, pioglitazone, and ranitidine. The document then provides background on nitrosamines and how they can be generated during drug manufacturing through use of reagents, catalysts, solvents or raw materials containing amines or nitrites. It outlines sources of nitrosamine impurities and recommends ways to prevent their formation, such as avoiding use of amines with nitrosating agents, properly storing materials, cleaning equipment, and modifying processes.