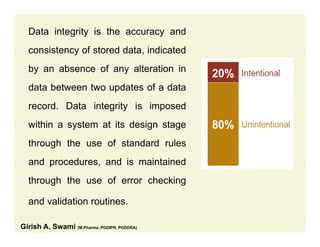
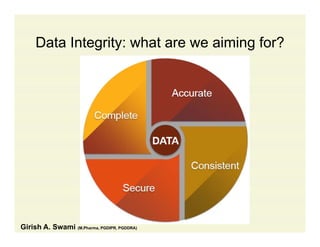
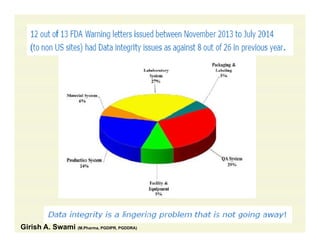
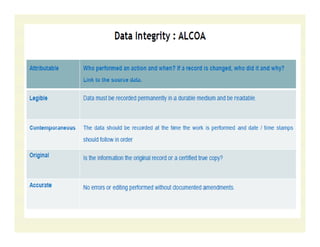
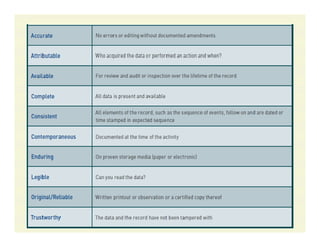
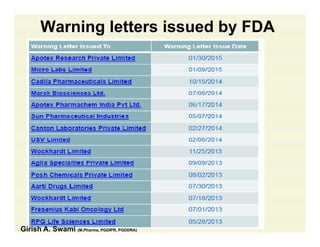
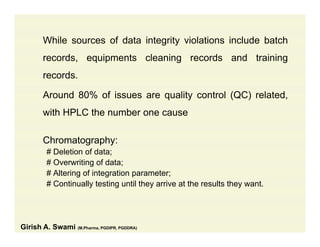
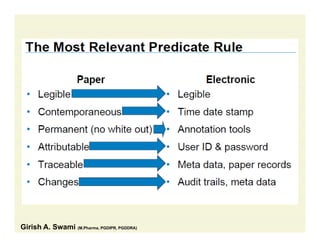
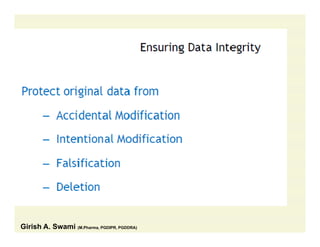
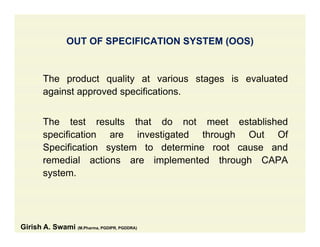
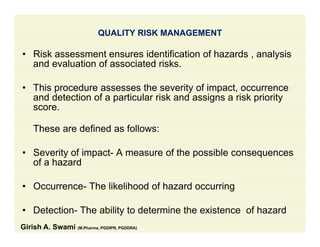
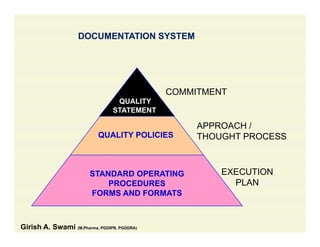
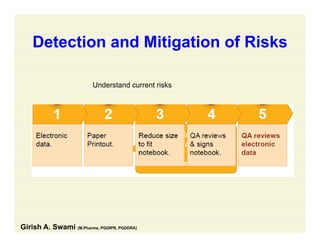
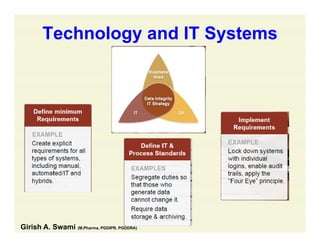
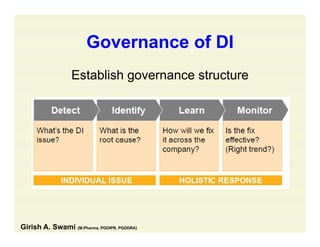
The document discusses data integrity and recent regulatory approaches. It defines data integrity as the completeness, consistency, and accuracy of data throughout its lifecycle. Regulatory agencies are increasingly focused on data integrity due to its importance in ensuring product quality and safety. Common data integrity issues found by agencies include fabricated, falsified, or missing records. Ensuring data integrity requires effective quality management systems, risk management, technology solutions, and governance.