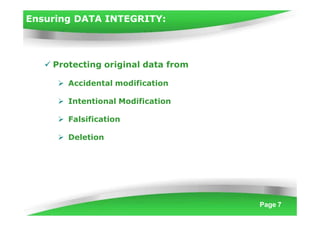
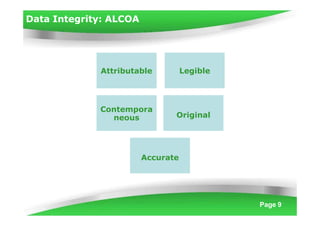
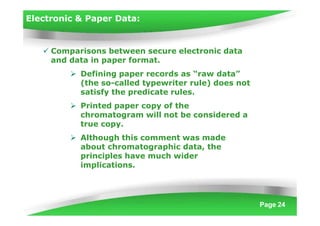
Data integrity is becoming increasingly important under cGMP regulations. Without reliable data, the quality of raw materials, in-process materials, and finished products cannot be ensured. Data integrity issues constitute both 21 CFR Part 11 and serious cGMP violations. If laboratory data integrity is compromised, products may not comply with regulatory terms or be released for sale. Regulatory agencies like the FDA have increased their focus on data integrity and reliability in recent years. Inspectors examine data based on multiple standards including cGMP, GLP, GCP, and data application integrity policies using a "guilty until proven innocent" approach.