- The document discusses data integrity, which refers to maintaining accurate and consistent data over its entire lifecycle. This is important for the regulated healthcare industry as quality decisions are based on data.
- The FDA uses the ALCOA criteria (Attributable, Legible, Contemporaneous, Original, Accurate) to define expectations for electronic data. Regulatory agencies now focus heavily on data integrity due to instances of fabricated documents and errors.
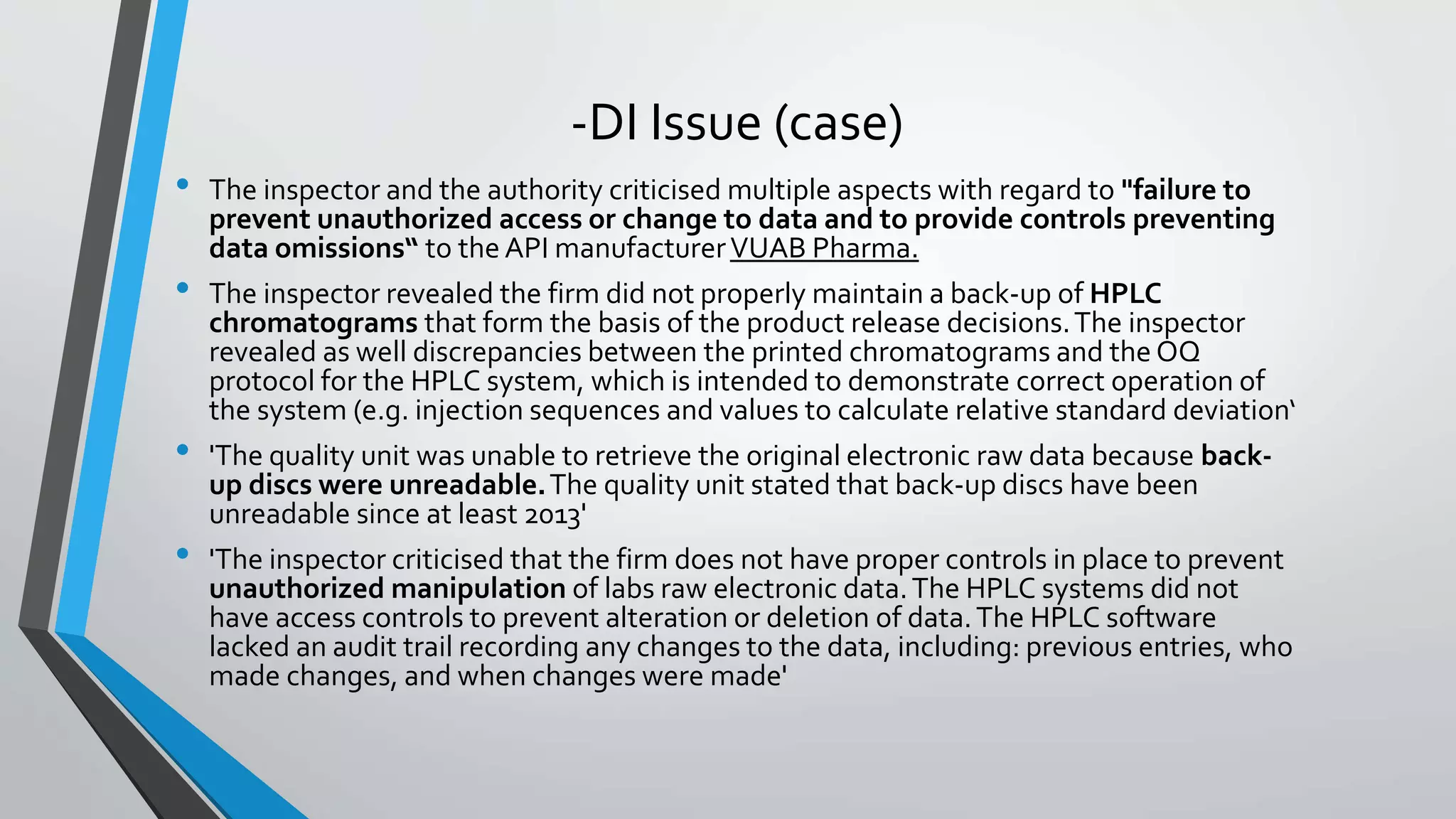
- Common data integrity issues found by agencies include non-contemporaneous recording, backdating records, re-running samples until desired results are obtained, and data fabrication. Ensuring data integrity helps prevent regulatory actions like warning letters or import bans against companies.