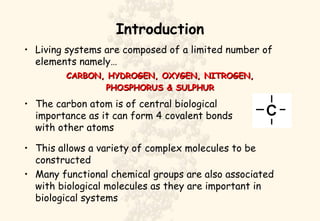
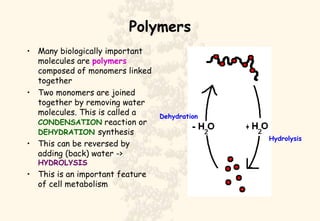
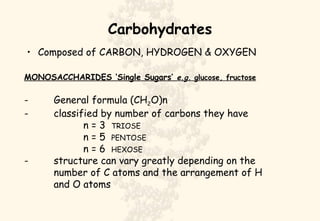
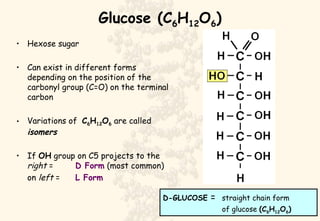
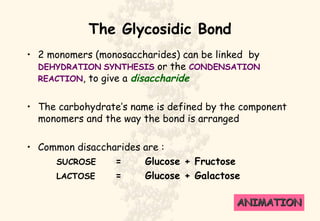
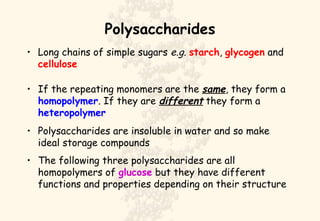
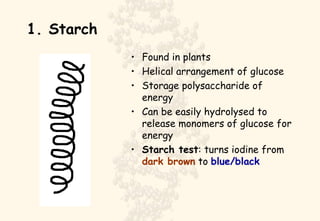
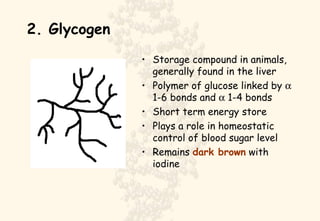
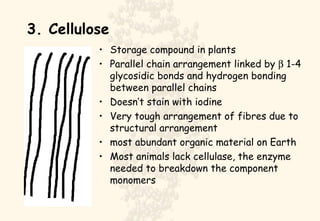
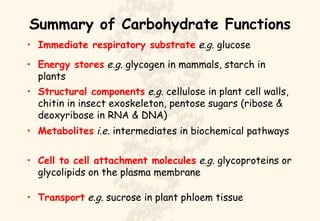
This document summarizes the structure and function of important cell components. It describes how carbon, hydrogen, oxygen, nitrogen, phosphorus and sulfur are used to form biologically important molecules like carbohydrates. Carbohydrates are polymers of simple sugars (monosaccharides) joined by dehydration reactions. They serve key functions like providing immediate energy through glucose, energy storage as starch and glycogen, structure as cellulose and chitin, and metabolism as intermediates. Important carbohydrates include monosaccharides, disaccharides, and polysaccharides.