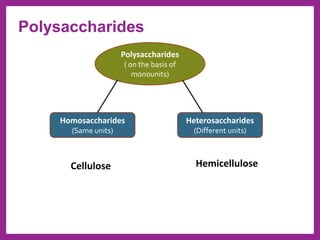
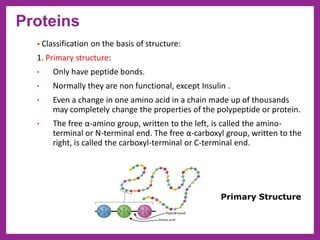
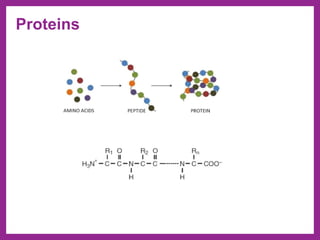
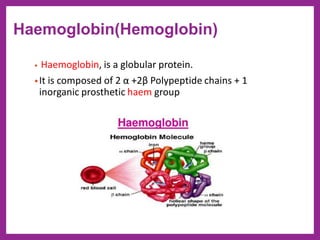
This document provides information on biological molecules. It begins by defining biomolecules as molecules involved in living organisms that are typically made up of carbon, hydrogen, oxygen, nitrogen and other elements. The main types of biomolecules discussed are carbohydrates, lipids, proteins, nucleic acids and water. Carbohydrates include monosaccharides like glucose and fructose, disaccharides like sucrose, and polysaccharides like starch, cellulose and glycogen. Lipids include fats, waxes, phospholipids, glycolipids and sterols. The properties of water that allow it to act as the universal solvent in living systems are also summarized.