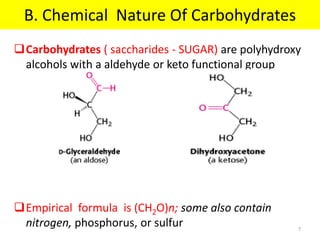
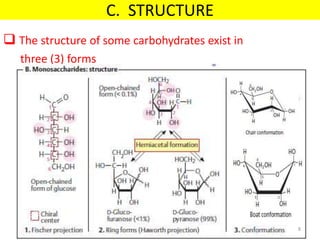
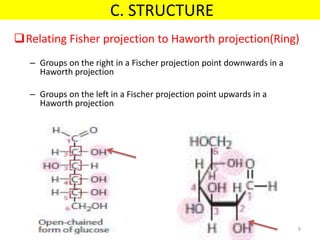
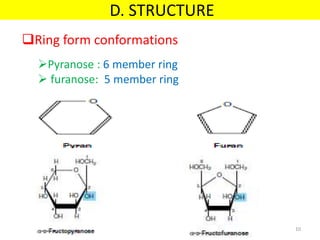
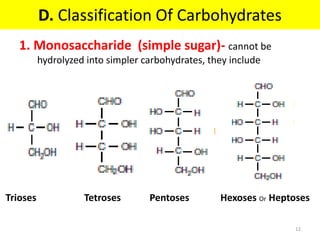
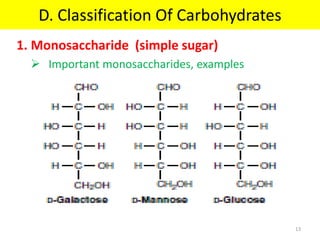
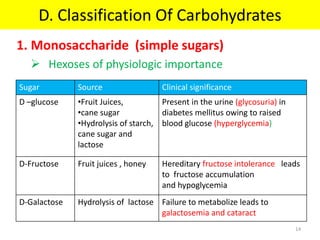
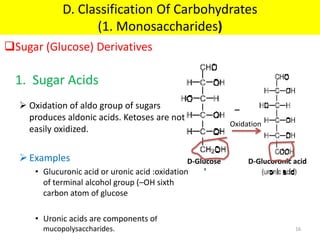
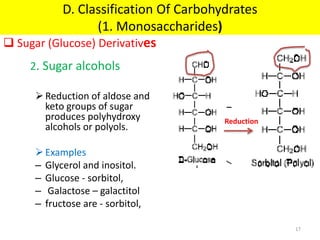
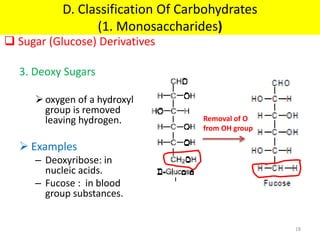
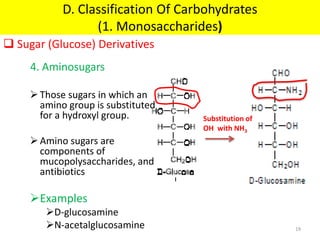
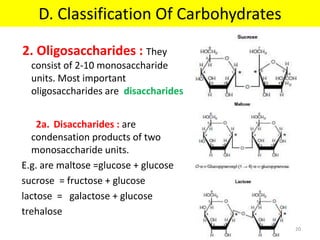
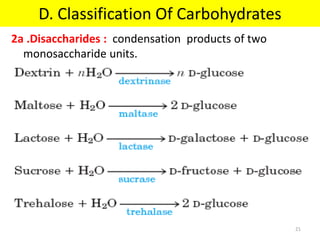
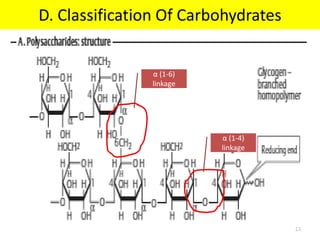
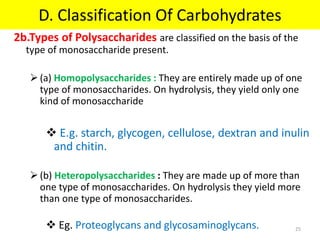
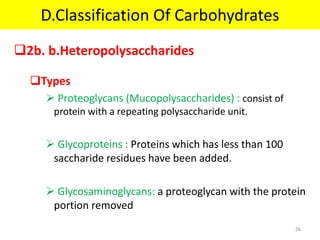
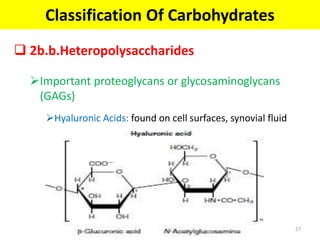
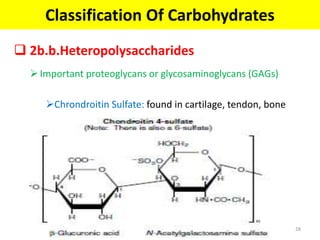
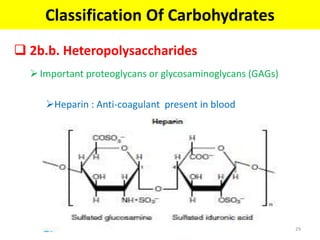
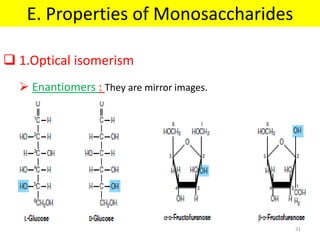
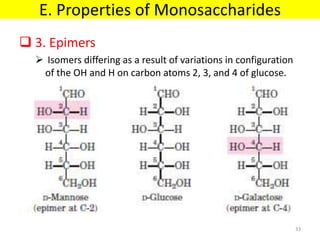
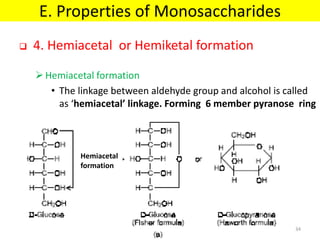
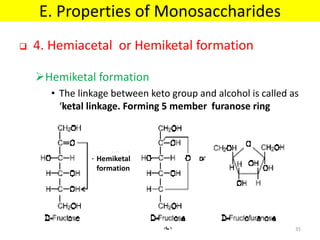
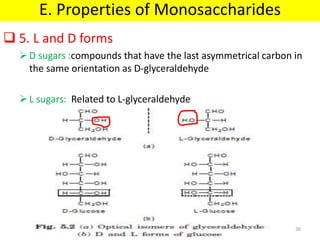
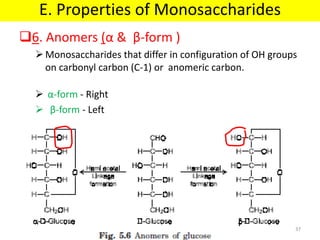
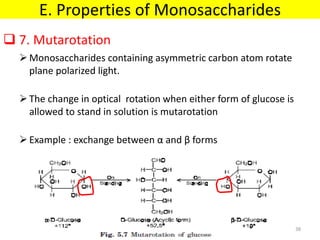
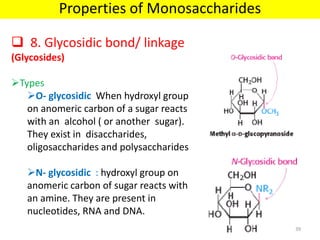
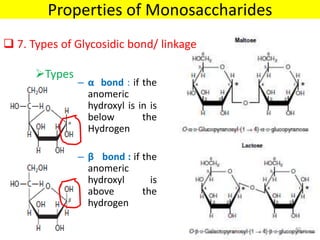
The document discusses carbohydrate structure and properties. It covers the biological and medical importance of carbohydrates, including their functions as energy stores and structural components. It also describes the chemical nature of carbohydrates as polyhydroxy alcohols with an aldehyde or keto group. Carbohydrate structure is examined using Fisher, Haworth and chair conformations. Carbohydrates are classified as monosaccharides, oligosaccharides like disaccharides, and polysaccharides including homo- and heteropolysaccharides. Important monosaccharides, derivatives, disaccharides and polysaccharides are identified. Properties of monosaccharides such as isomerism, optical activity, epimerism, hemiacetal/ketal formation,