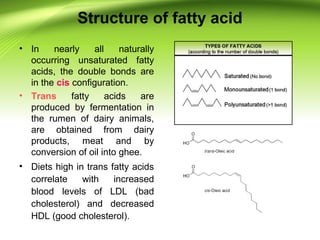
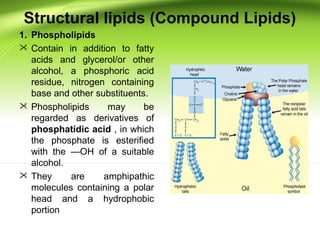
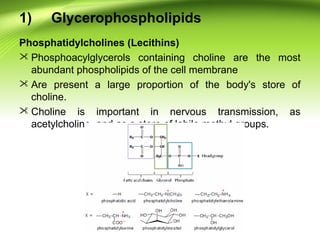
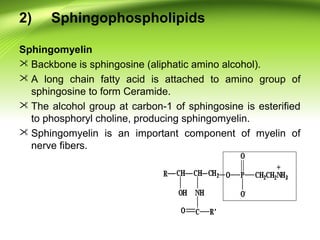
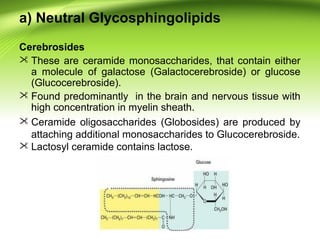
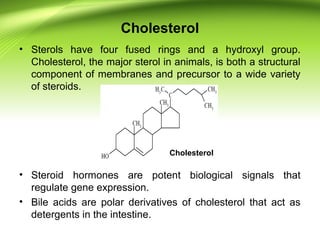
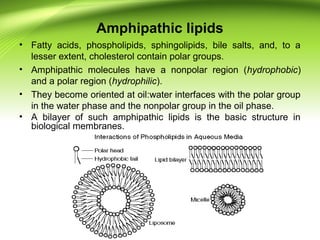
The lipids are a heterogeneous group of compounds that include fats, oils, steroids, waxes, and related compounds. They share the properties of being relatively insoluble in water but soluble in nonpolar solvents. Lipids serve important functions such as energy storage, structural components of cell membranes, and insulation. They can also be classified based on their structure into simple lipids like triglycerides and complex lipids like phospholipids and glycolipids. Phospholipids and glycolipids are amphipathic molecules that form lipid bilayers, which are the basic structure of biological membranes.