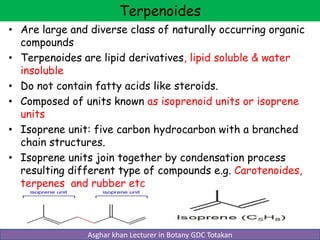
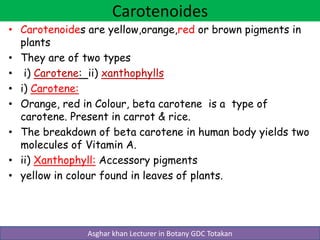
Lipids are a diverse group of organic compounds that contain carbon, hydrogen, oxygen, nitrogen and phosphorus. They are non-polar and hydrophobic, meaning they are insoluble in water but soluble in organic solvents. Lipids include triglycerides, phospholipids, waxes, steroids, and terpenoids. They serve important structural and energy storage functions in living organisms.