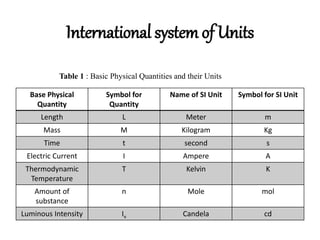
Chemistry is the study of matter and its composition, structure, properties, and interactions. [1] Some key concepts introduced in the document include the classification of matter as elements, compounds, or mixtures based on its chemical and physical properties. [2] Five important laws governing chemical combinations are also outlined: the law of conservation of mass, the law of definite proportions, the law of multiple proportions, Gay-Lussac's law of gaseous volumes, and Avogadro's law. [3]