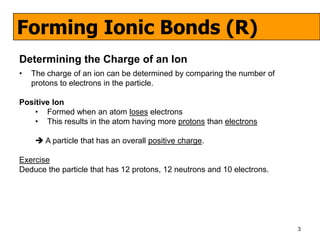
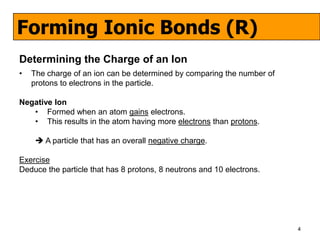
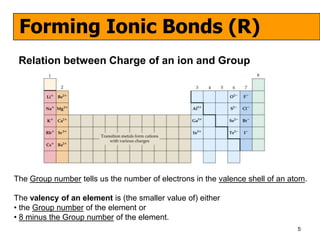
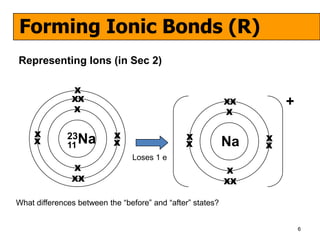
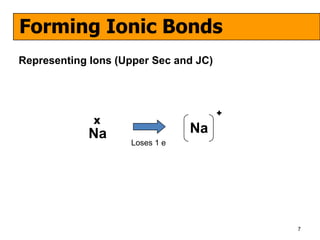
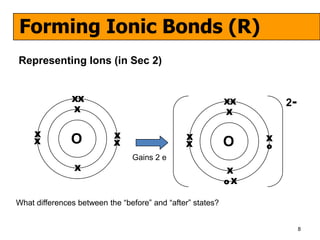
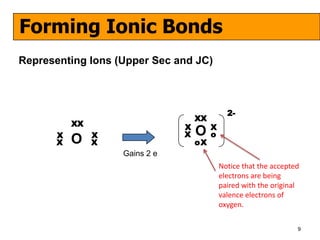
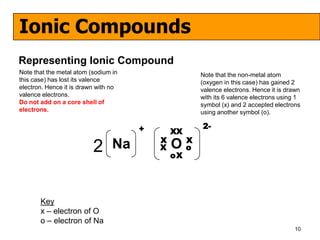
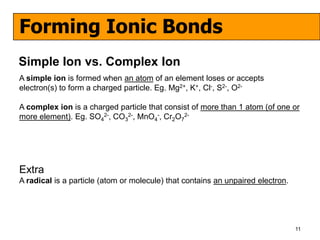
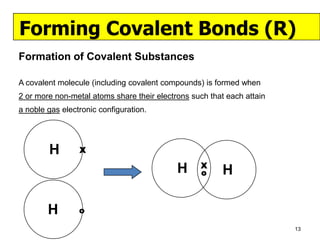
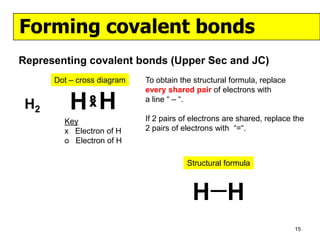
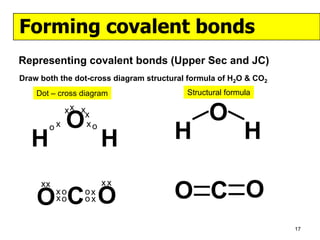
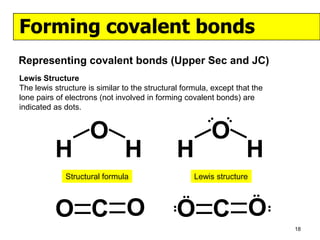
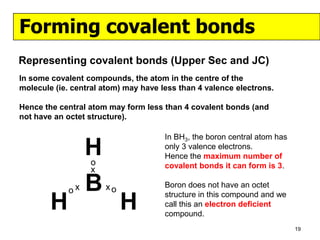
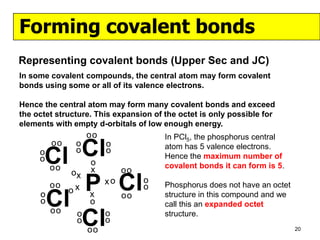
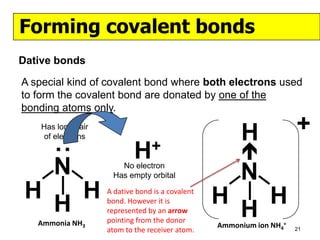
The document discusses the formation of ionic and covalent bonds, including how to determine the charge of ions, represent ionic compounds using dot-cross diagrams, and represent covalent molecules using dot-cross diagrams, structural formulas, and Lewis structures to show how atoms share electrons to achieve stable configurations. It also discusses exceptions where central atoms in some covalent compounds have fewer than 8 valence electrons.