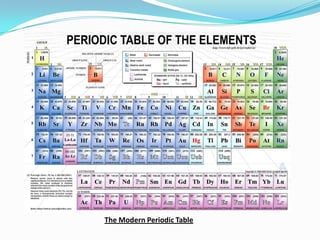
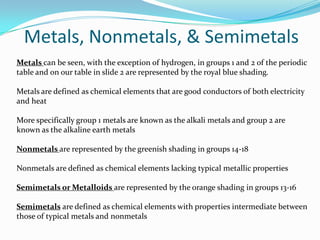
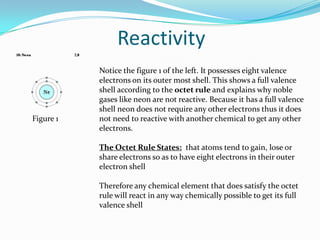
This document provides an introduction to the periodic table, including its history, structure, and trends. It describes how Dmitri Mendeleev organized the elements into groups and periods based on their properties, with metals on the left, nonmetals on the right, and noble gases at the bottom. Groups are arranged horizontally and periods vertically. The location of an element determines its properties, like reactivity and atomic radius, following periodic trends. The periodic table is an important tool in chemistry that continues to be refined.