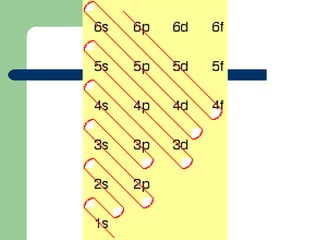
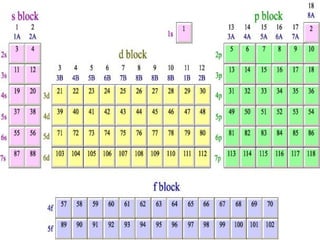
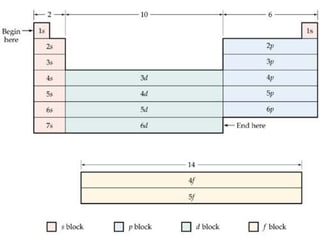
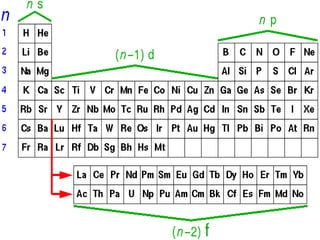
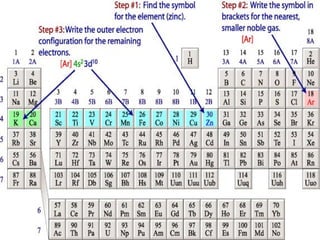
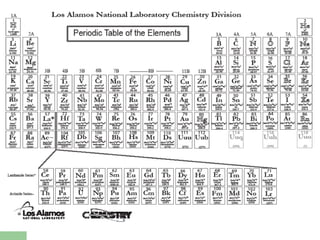
The document provides a review of key concepts about periodic blocks and electron configurations. It discusses the s, p, d, and f blocks and how they relate to the periodic table structure. The main points covered are:
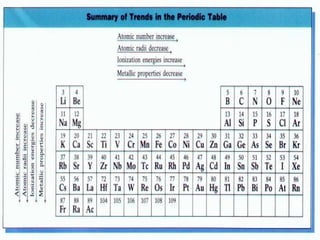
- Elements are organized by period and group based on their valence electrons and orbital occupation
- The s block contains groups 1 and 2 and fills the s orbital
- The p block contains groups 3-8 and fills the p orbital
- The d block contains the transition metals and fills the d orbital
- The f block contains the lanthanides and actinides and fills the f orbital














![Practice problems!!! Periodic tables away!!! Tell me the group, period, and block of an atom with the following electron configurations: [Ne]3s 2 [He]2s 2 [Kr]5s 2 4d 10 5p 5](https://image.slidesharecdn.com/periodic-block-presentation-120189564236987-4/85/Periodic-Block-Presentation-22-320.jpg)
![Answers [Ne]3s 2 Group 2a Period 3 S-block [He]2s 2 Group 2a Period 2 S-block [Kr]5s 2 4d 10 5p 5 Group 7a Period 5 p-block](https://image.slidesharecdn.com/periodic-block-presentation-120189564236987-4/85/Periodic-Block-Presentation-23-320.jpg)

![Answers The group 4b element in the fifth period? Zirconium [Kr]5s 2 4d 2 The group 1a element in the seventh period? Francium [Rn]7s 1](https://image.slidesharecdn.com/periodic-block-presentation-120189564236987-4/85/Periodic-Block-Presentation-25-320.jpg)