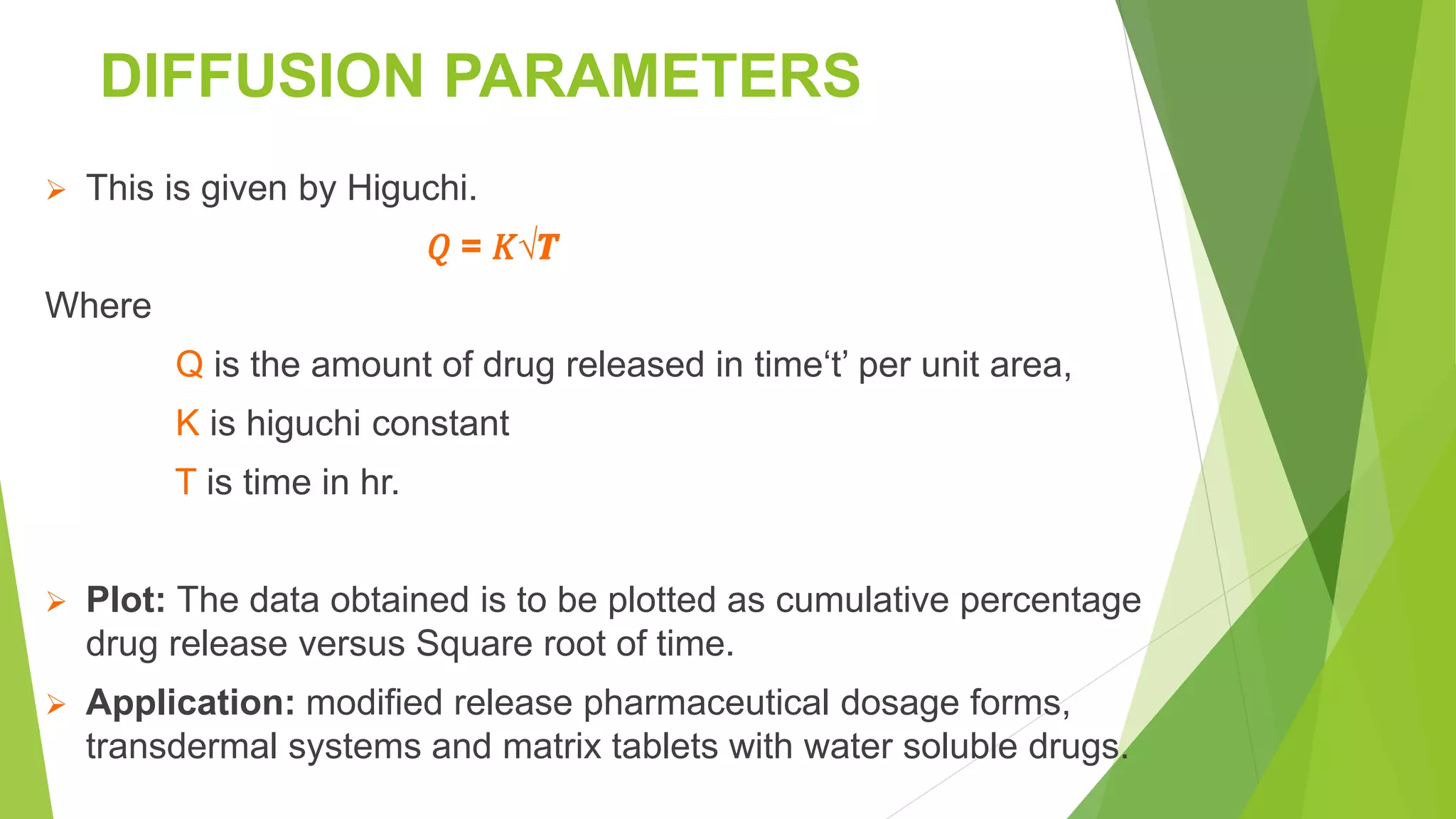
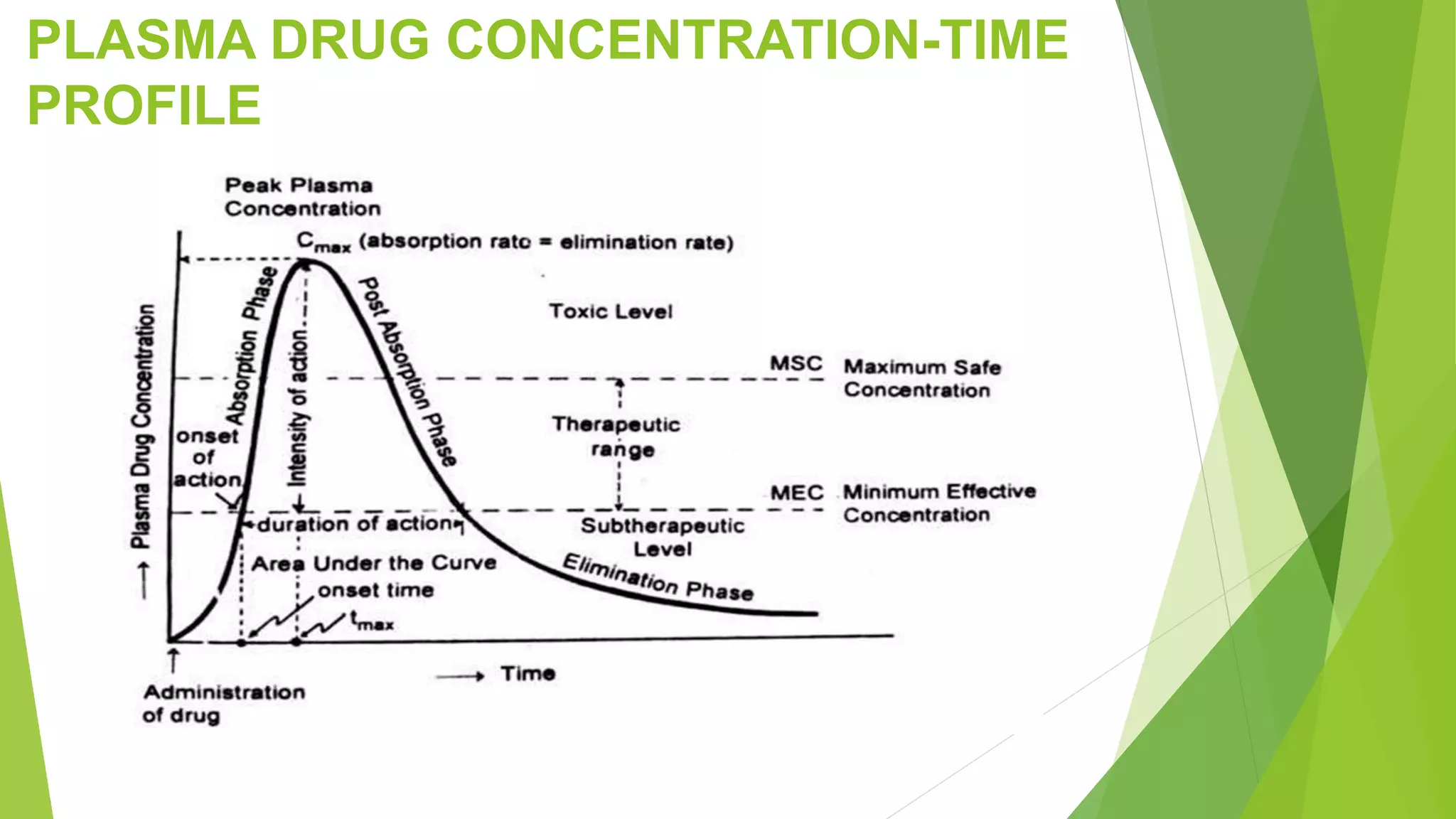
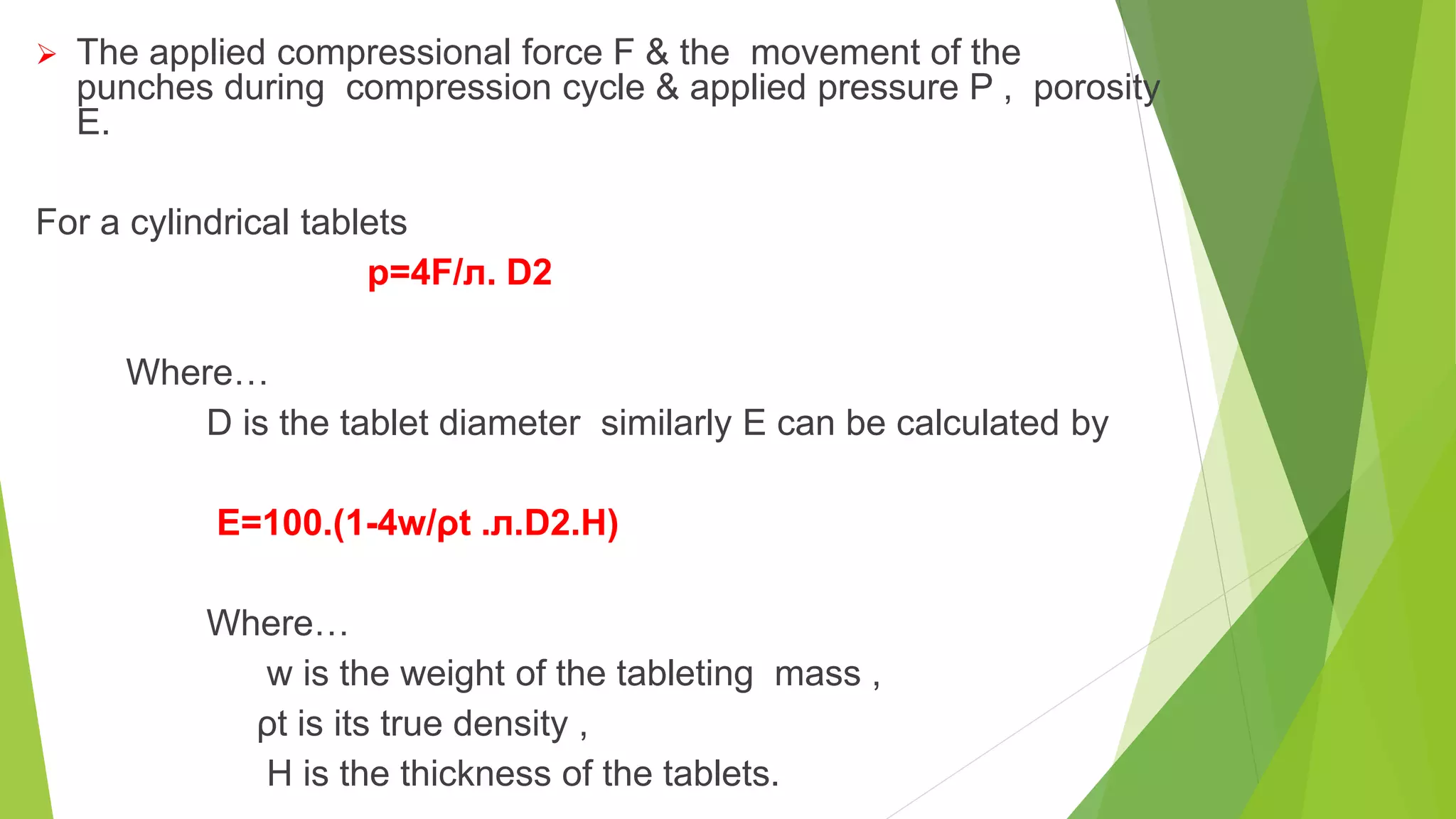
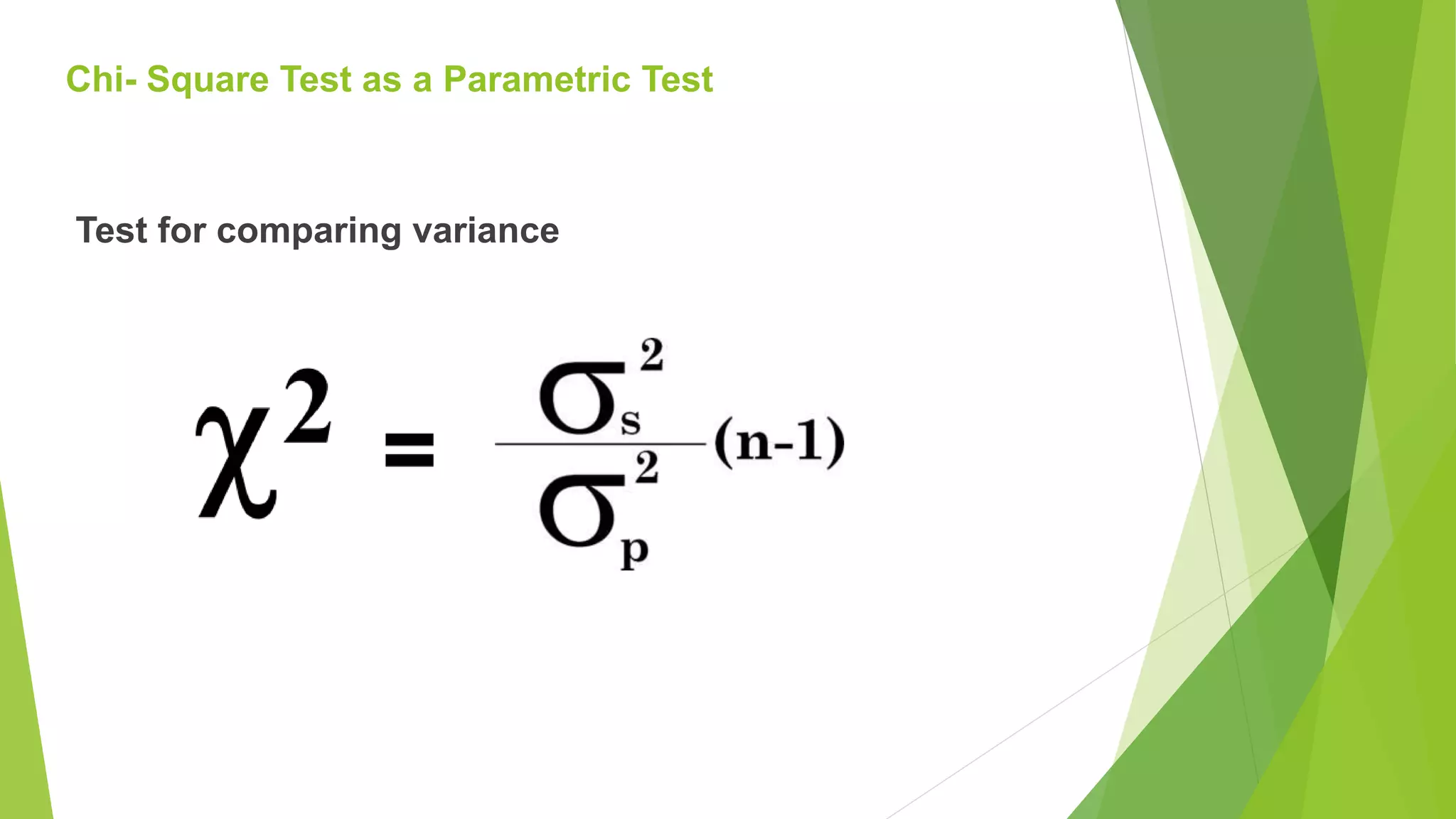
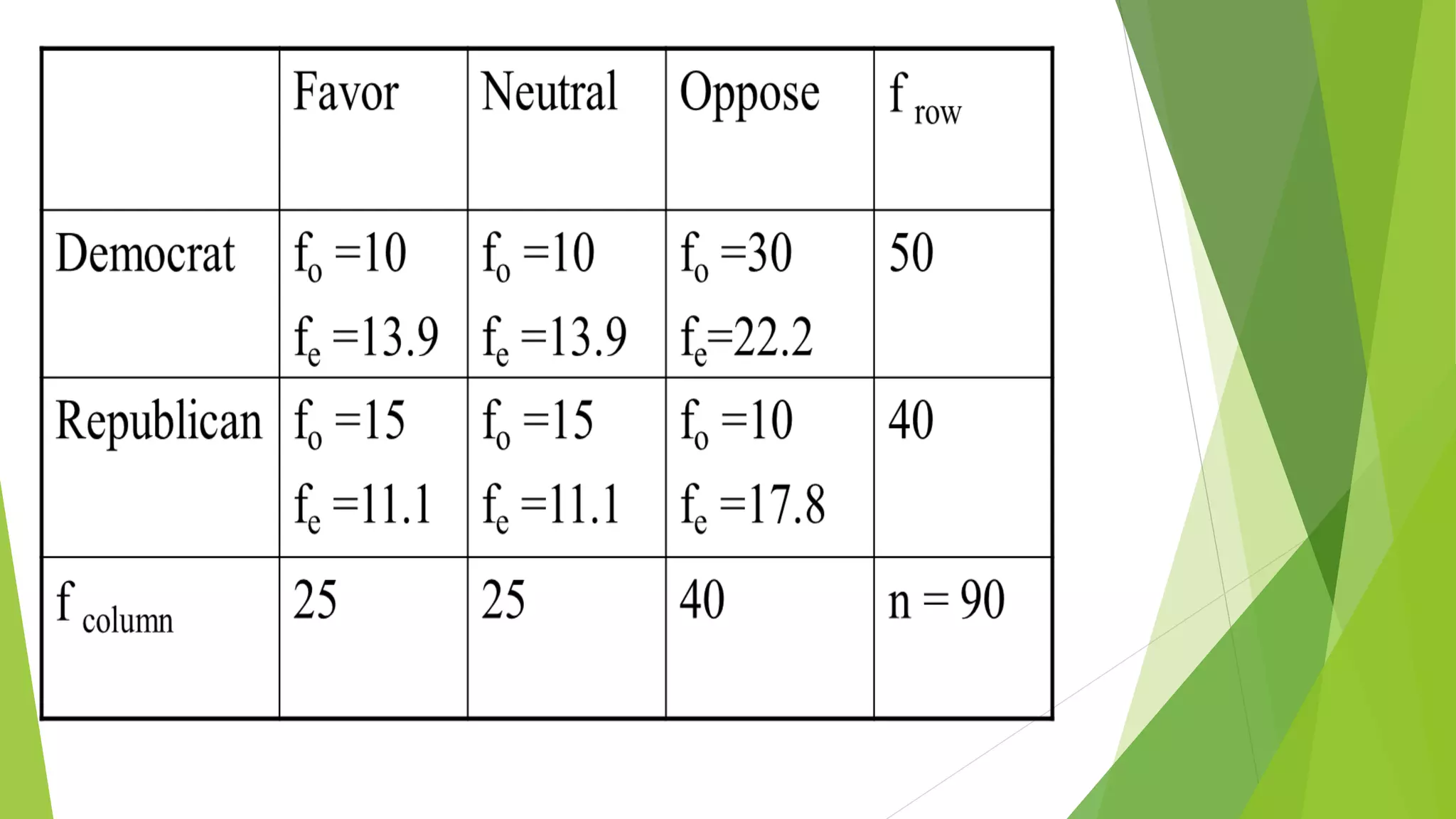
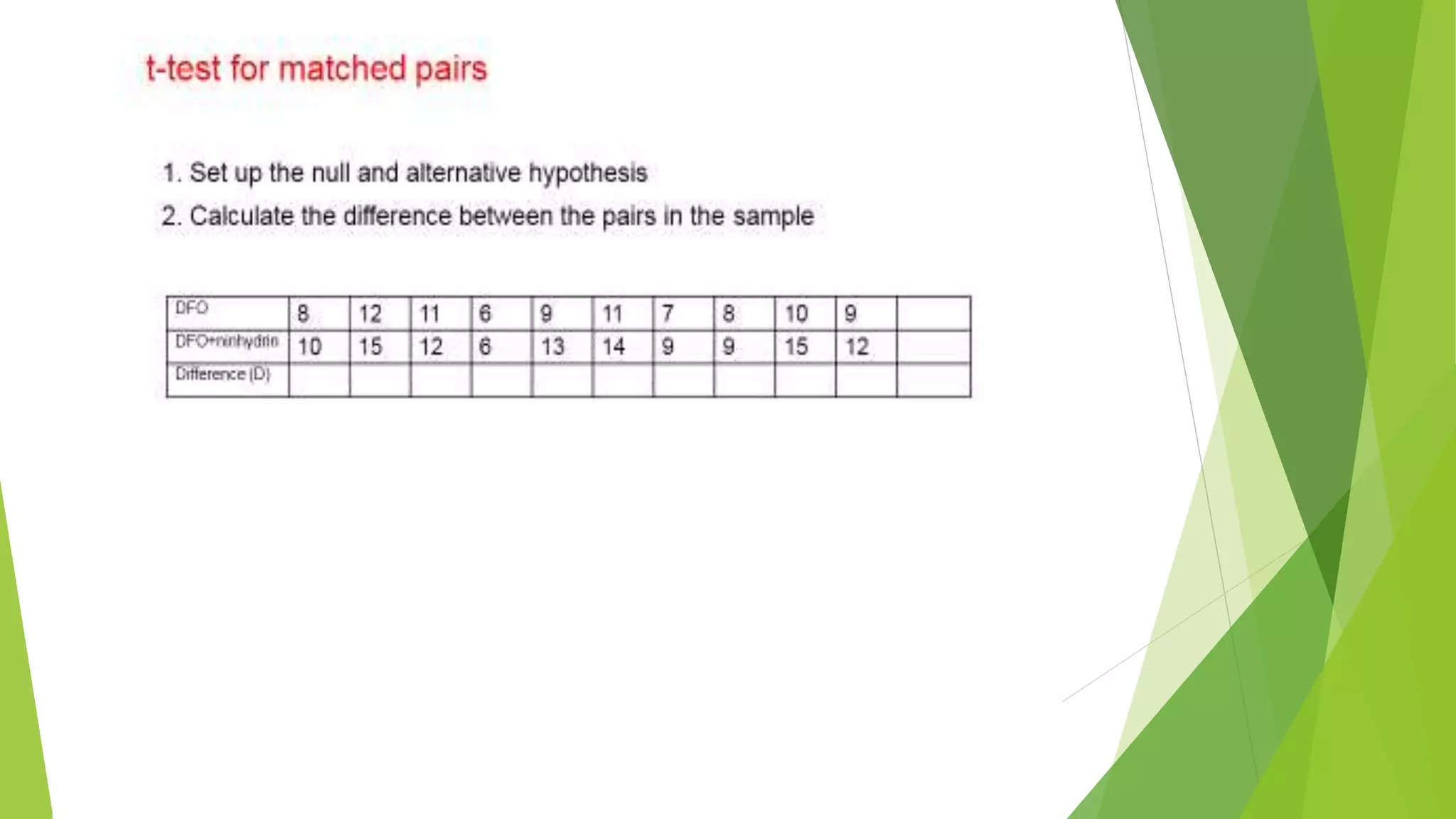
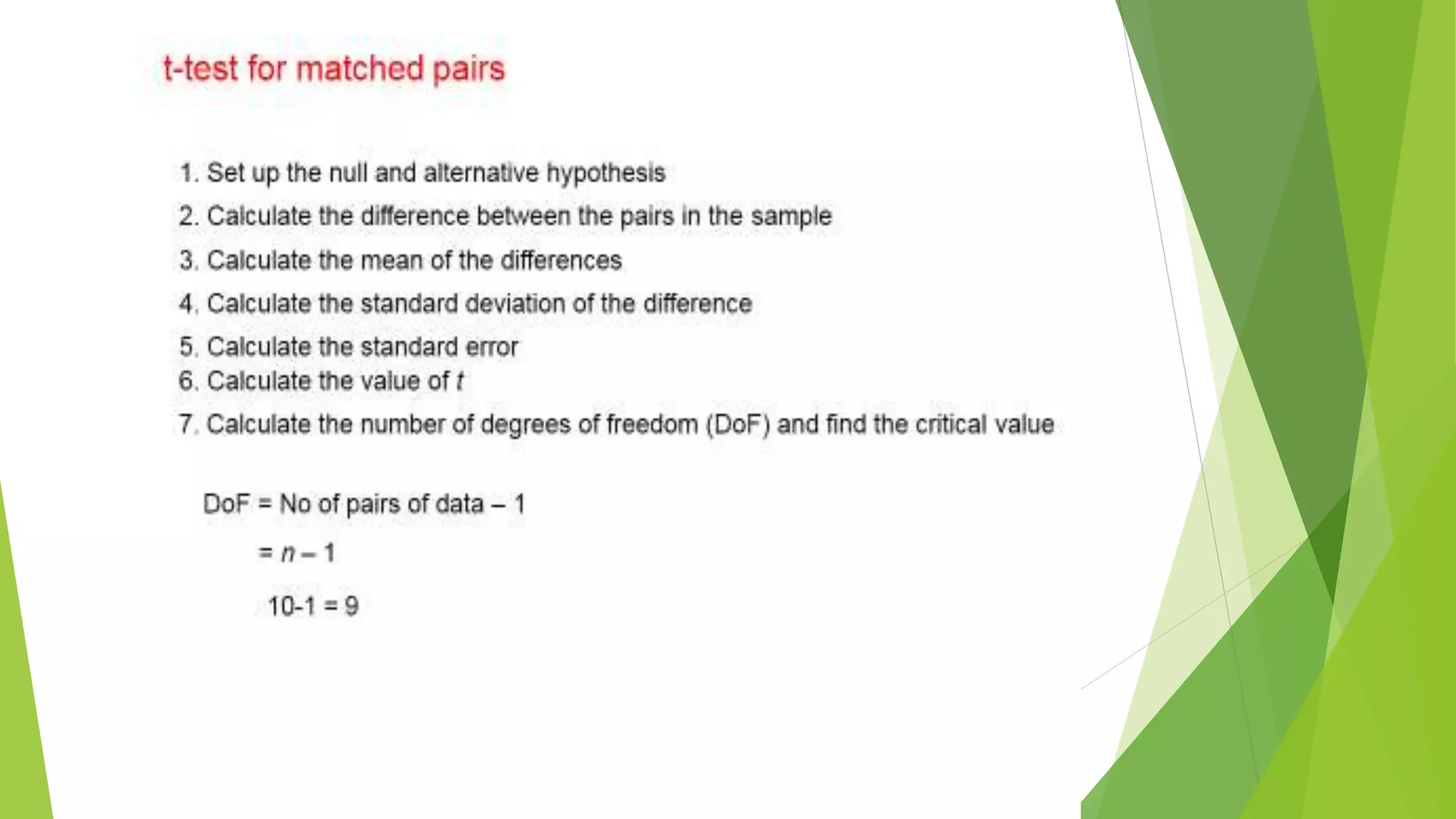
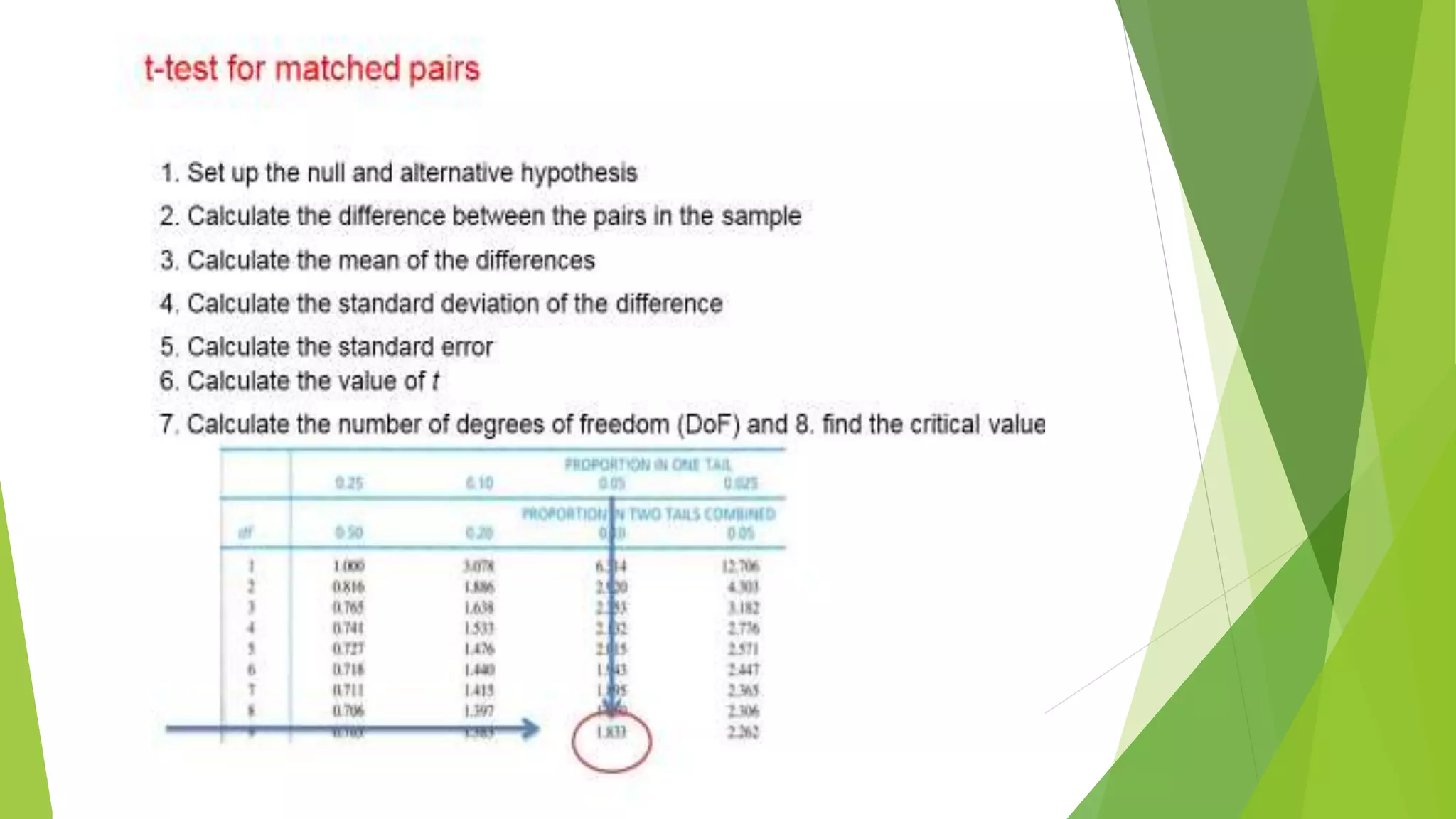
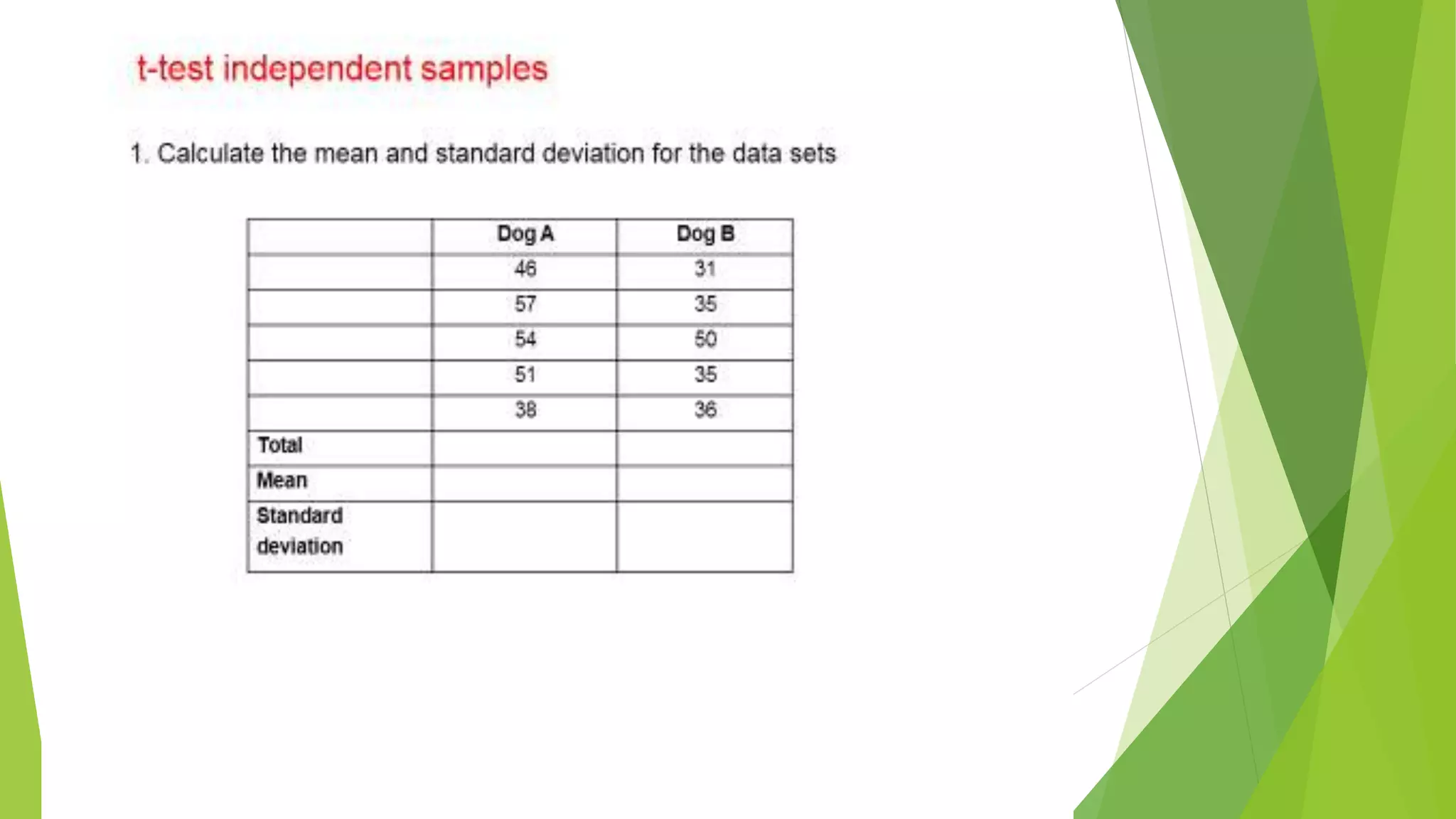
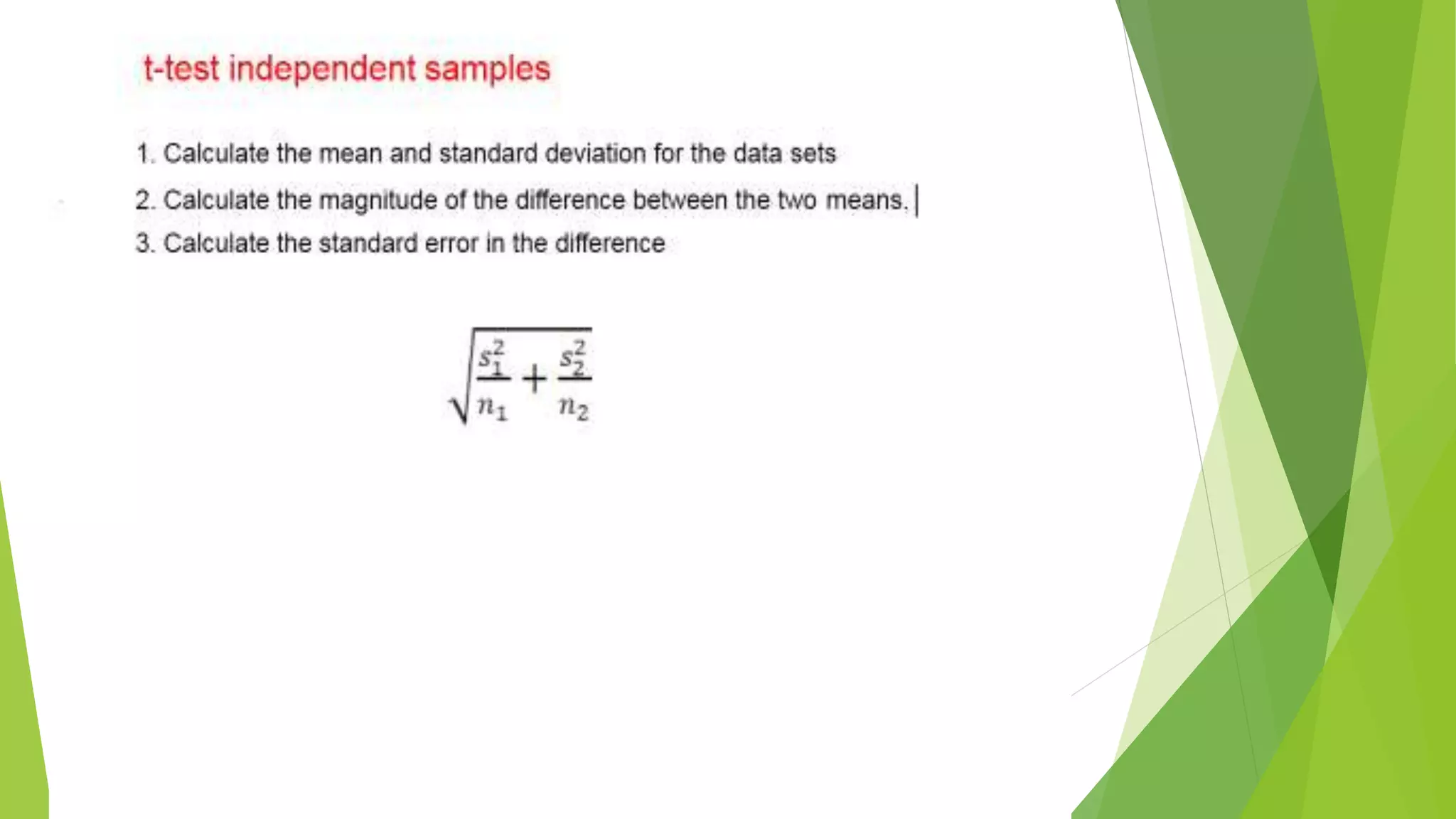
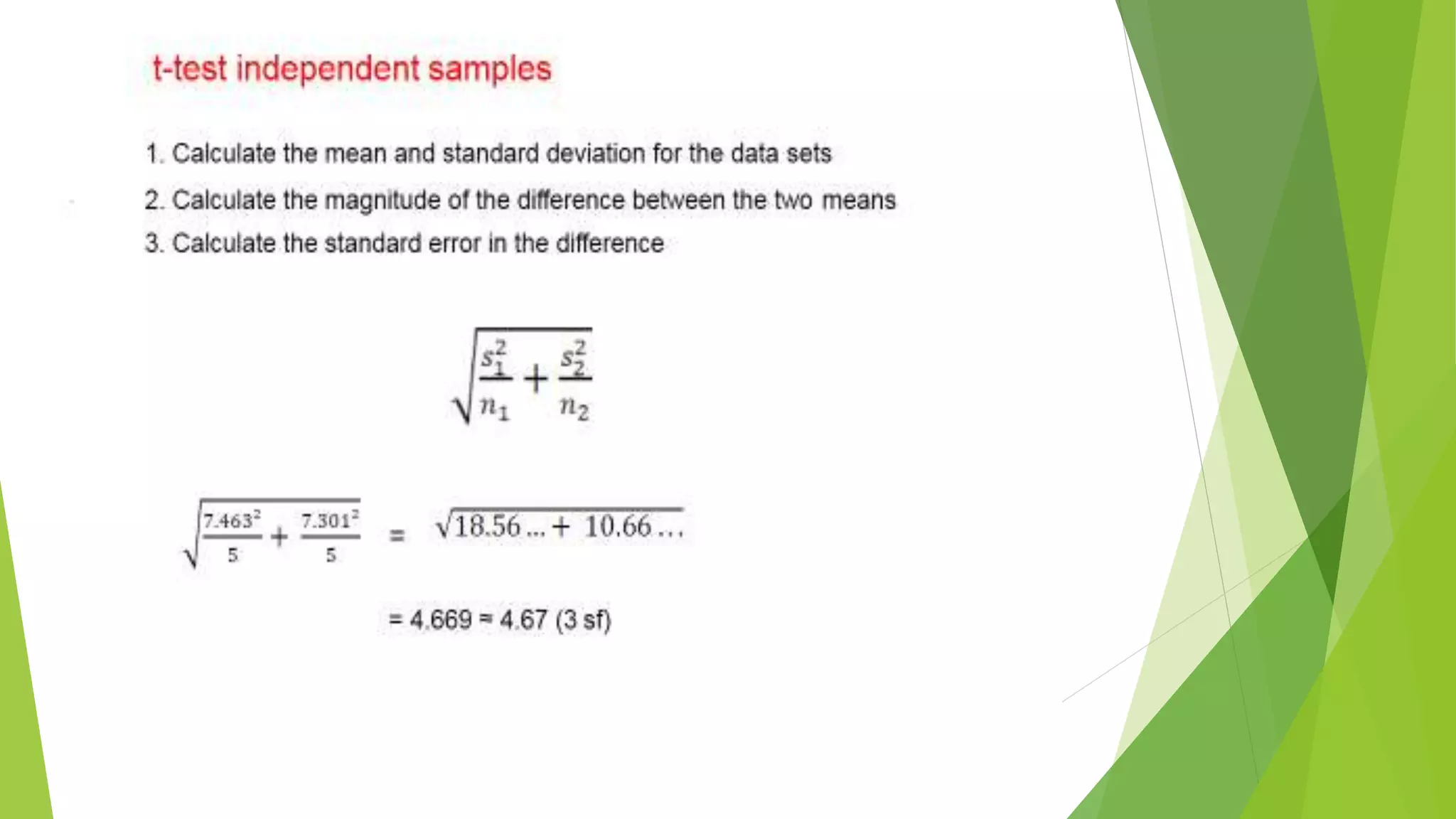
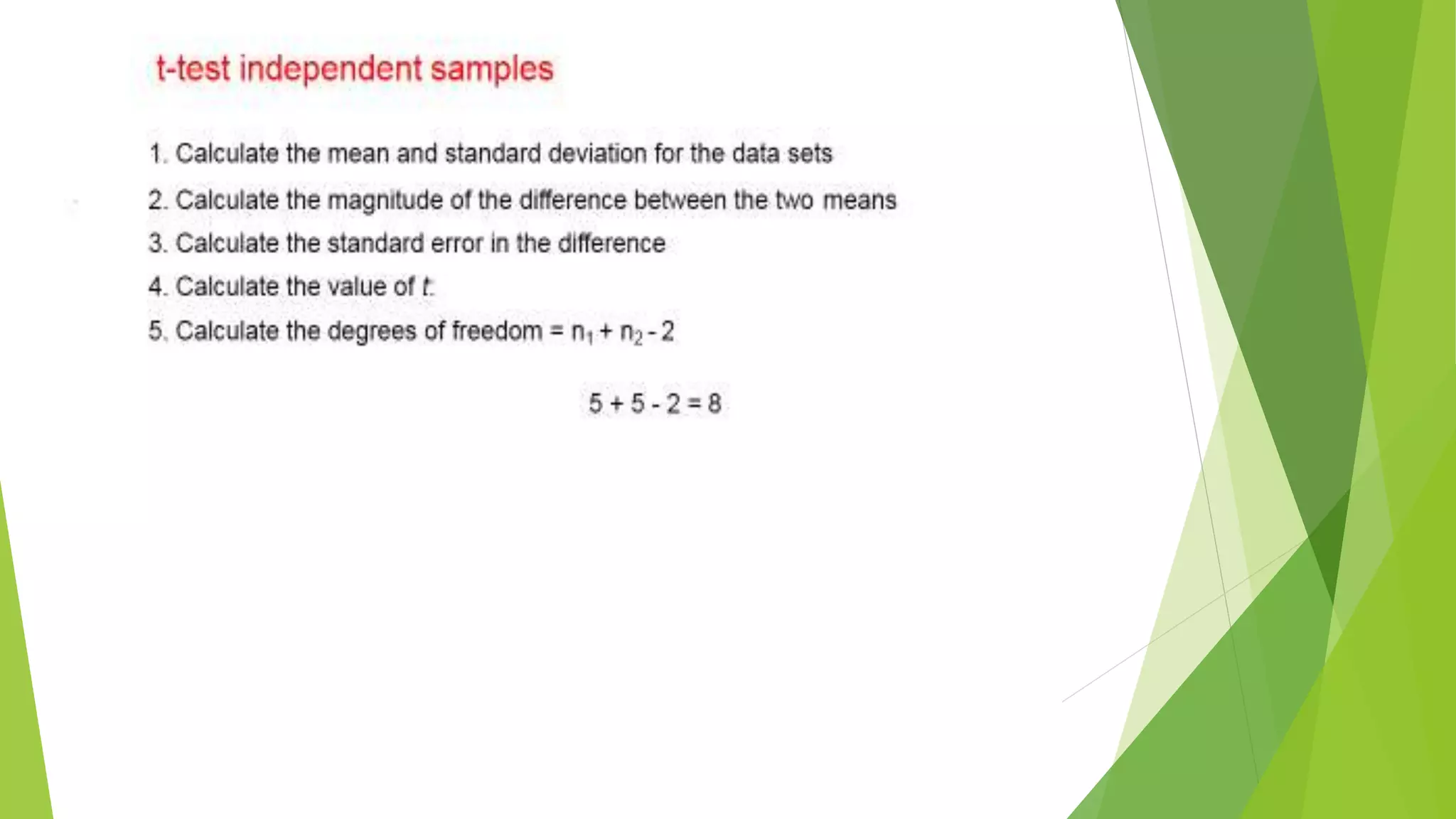
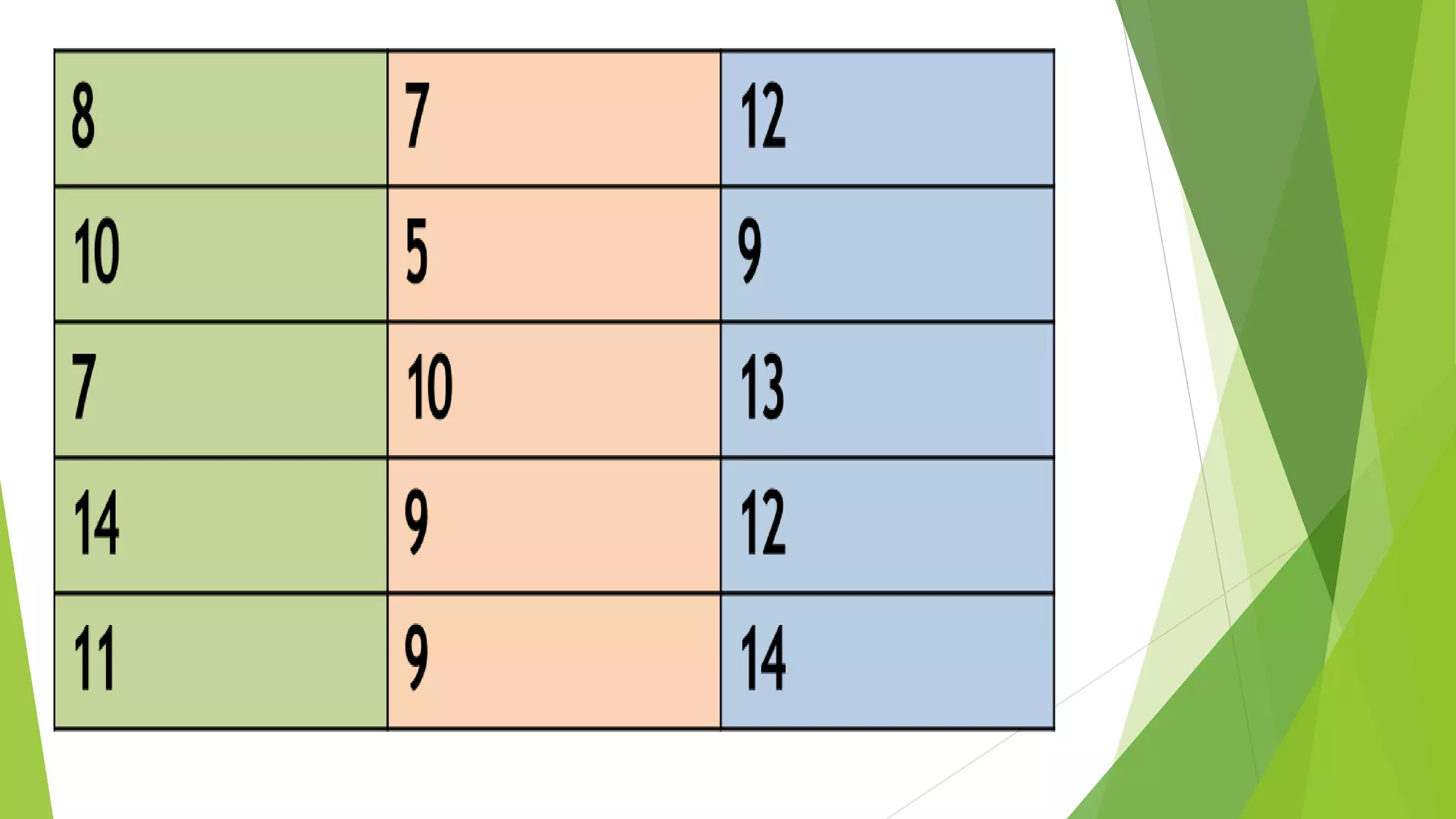
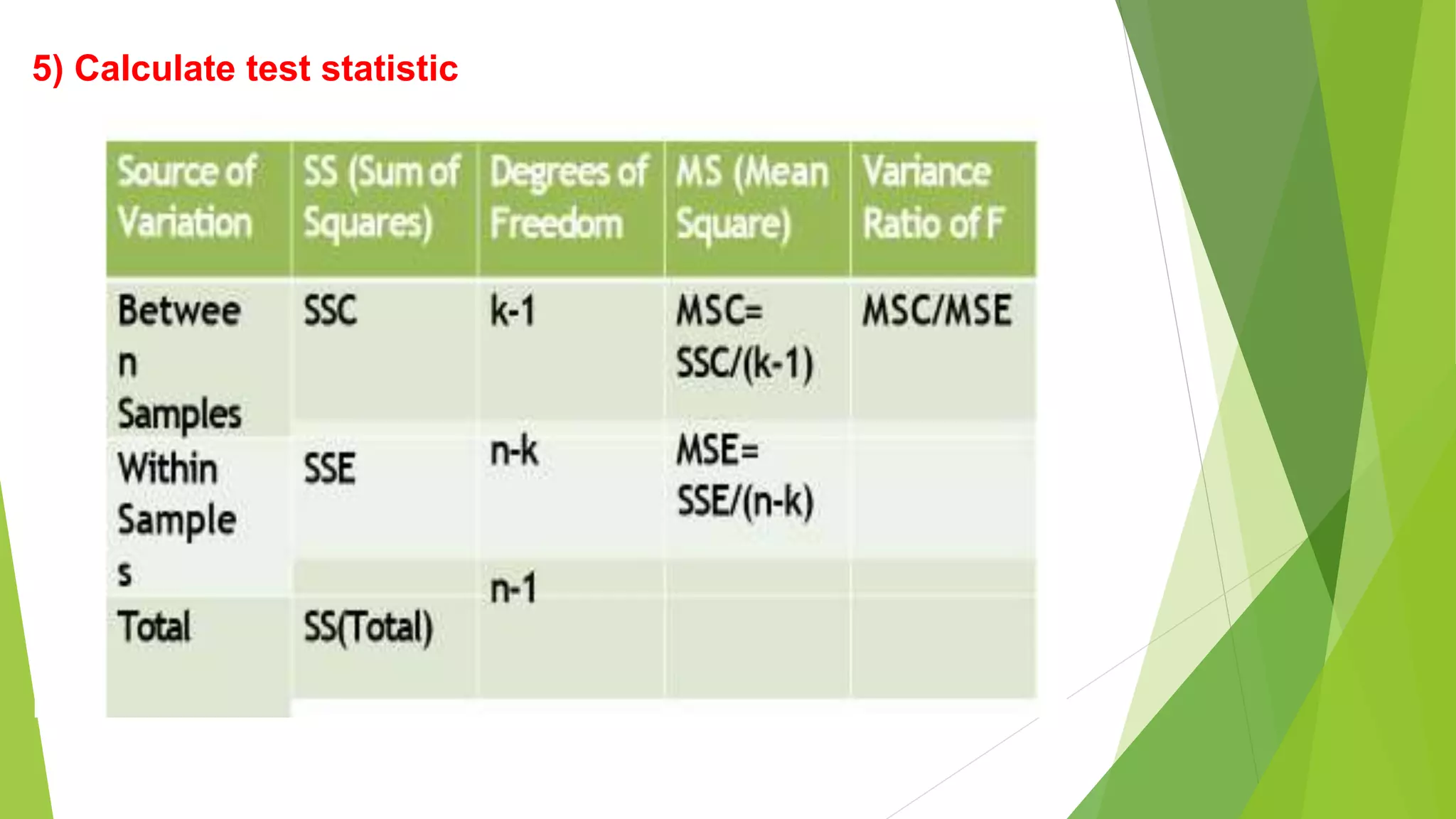
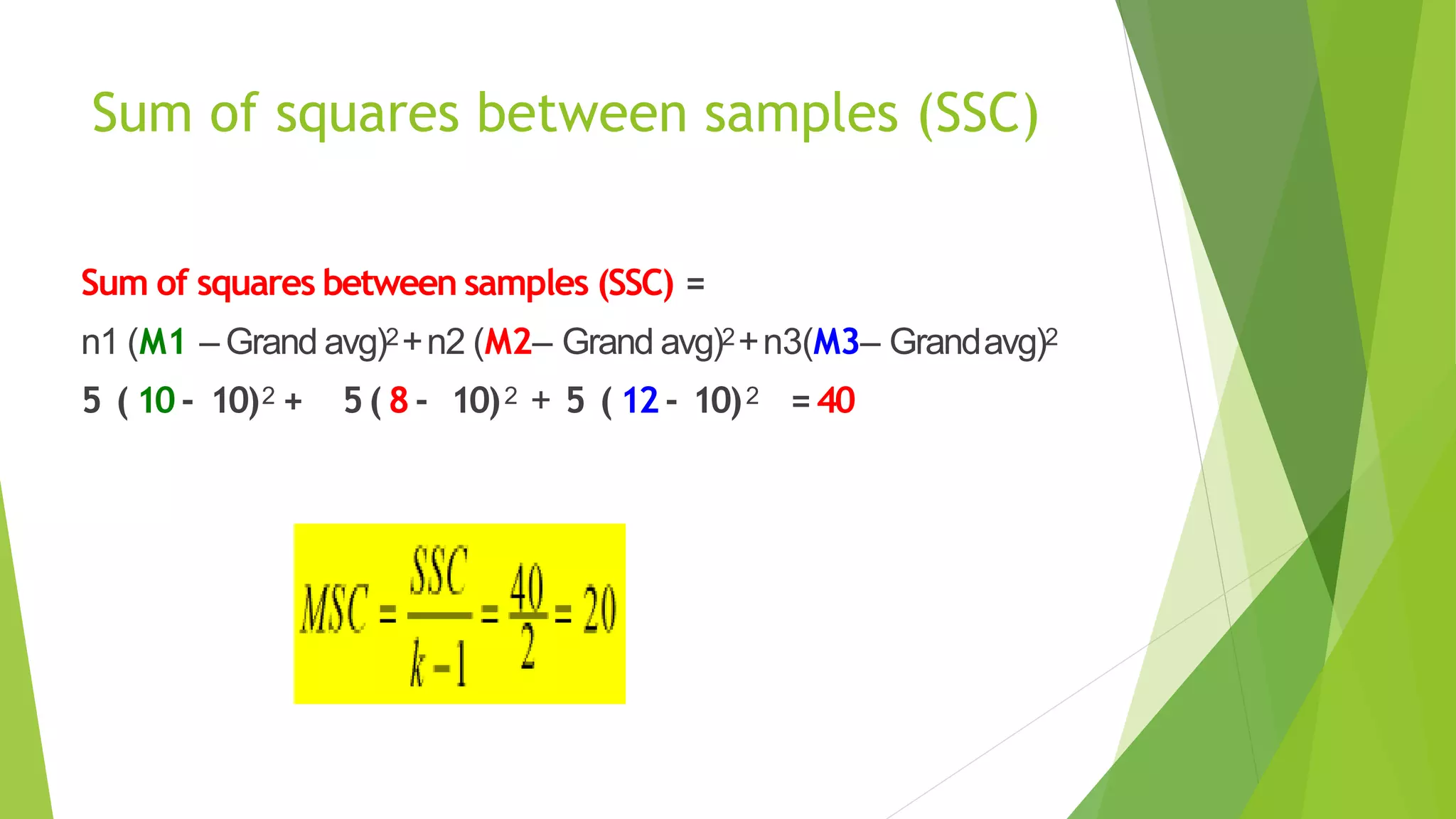
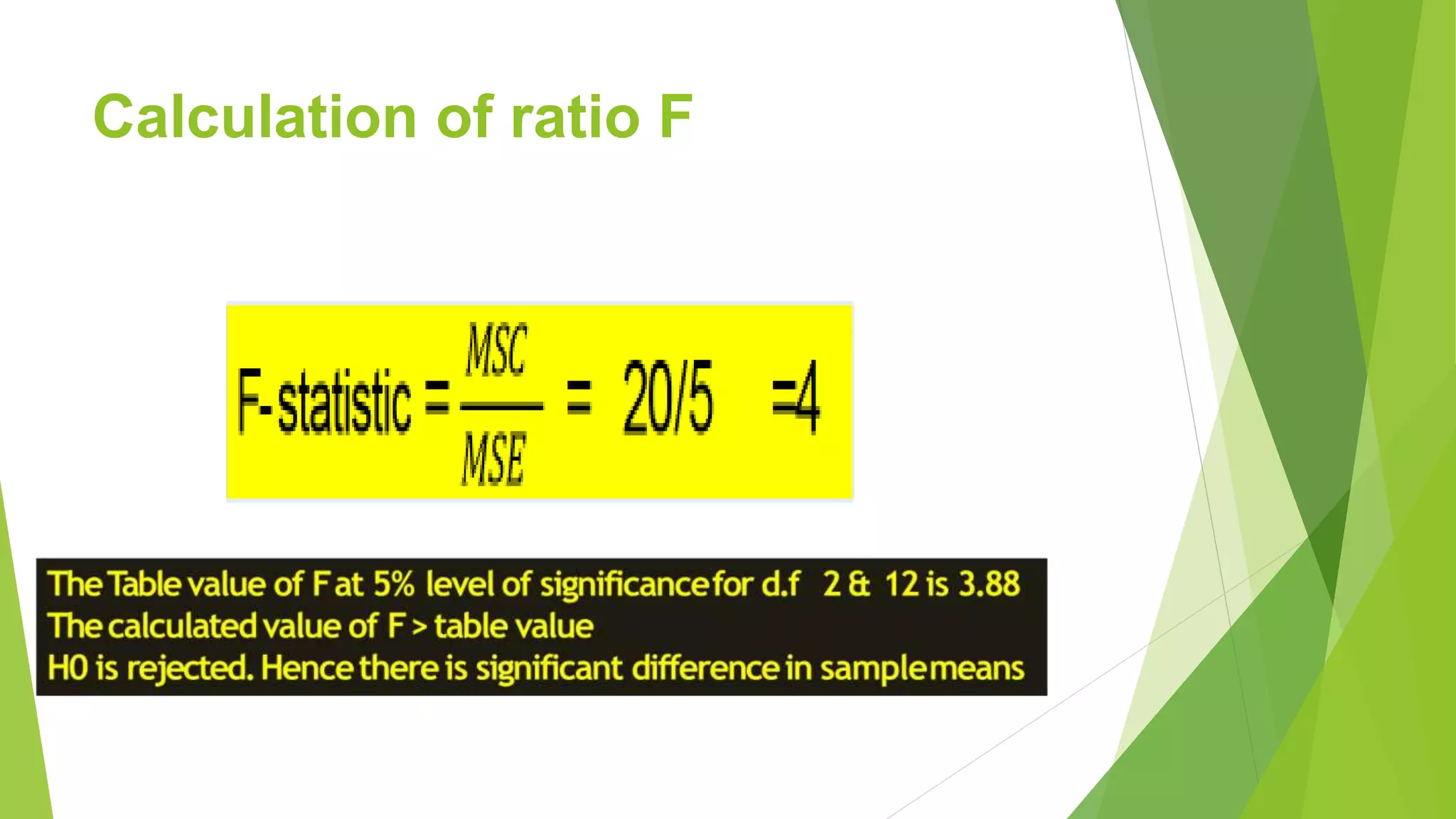
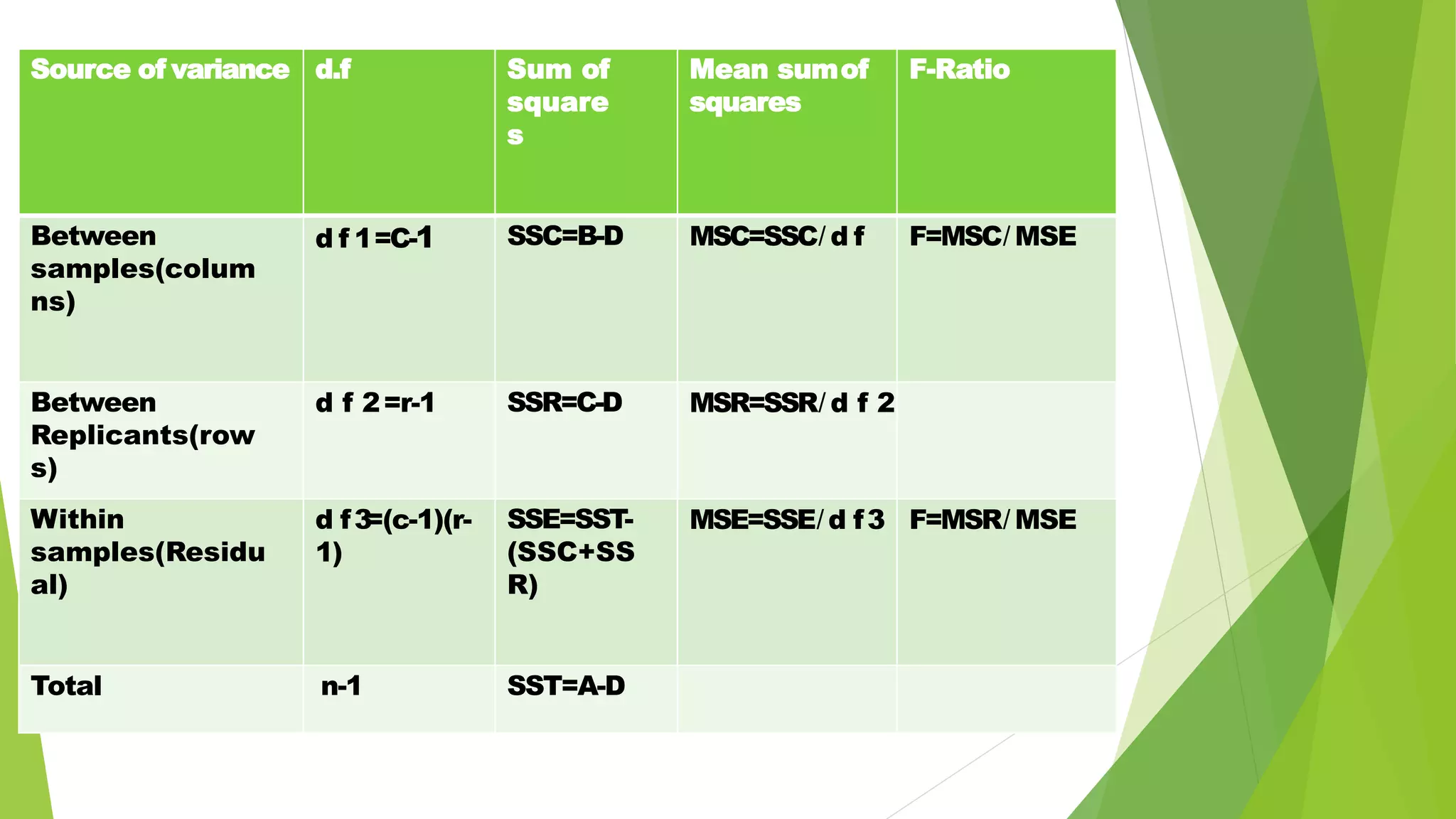
The document focuses on consolidation parameters in pharmaceutical sciences, detailing processes such as cold welding and fusion bonding, alongside various mechanisms impacting mechanical strength. It also explores diffusion and dissolution parameters critical for drug release, including effects of agitation, viscosity, and pH. Additionally, it covers pharmacokinetics, statistical methods including chi-square tests, student’s t-test, and ANOVA for analyzing data relevant to drug formulation and efficacy.