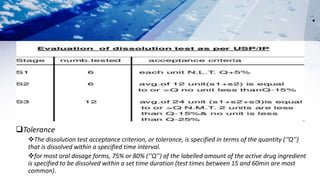
The document discusses drug product performance evaluation through in vitro dissolution testing. It provides details on factors that influence drug dissolution like drug substance properties, formulation composition, manufacturing process, and dissolution test conditions. The key goals of in vitro drug product testing are to characterize drug potency and release rate from oral dosage forms, provide information for formulation development, and ensure quality, comparability and stability over time. Common tests include disintegration testing and dissolution testing using apparatus specified in pharmacopeias to simulate gastrointestinal conditions. The results of in vitro testing aid product development and assessment of shelf-life and quality.