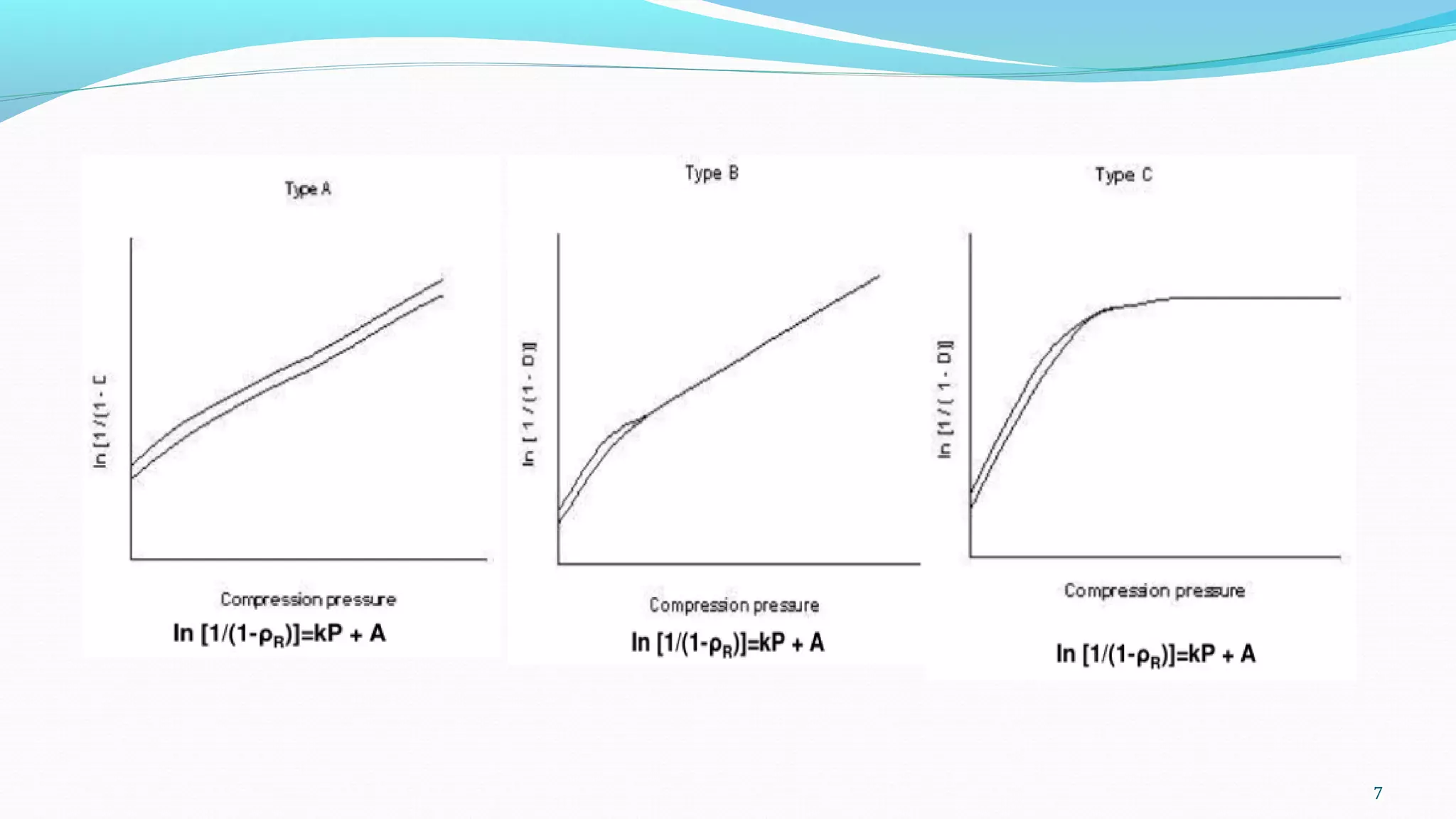
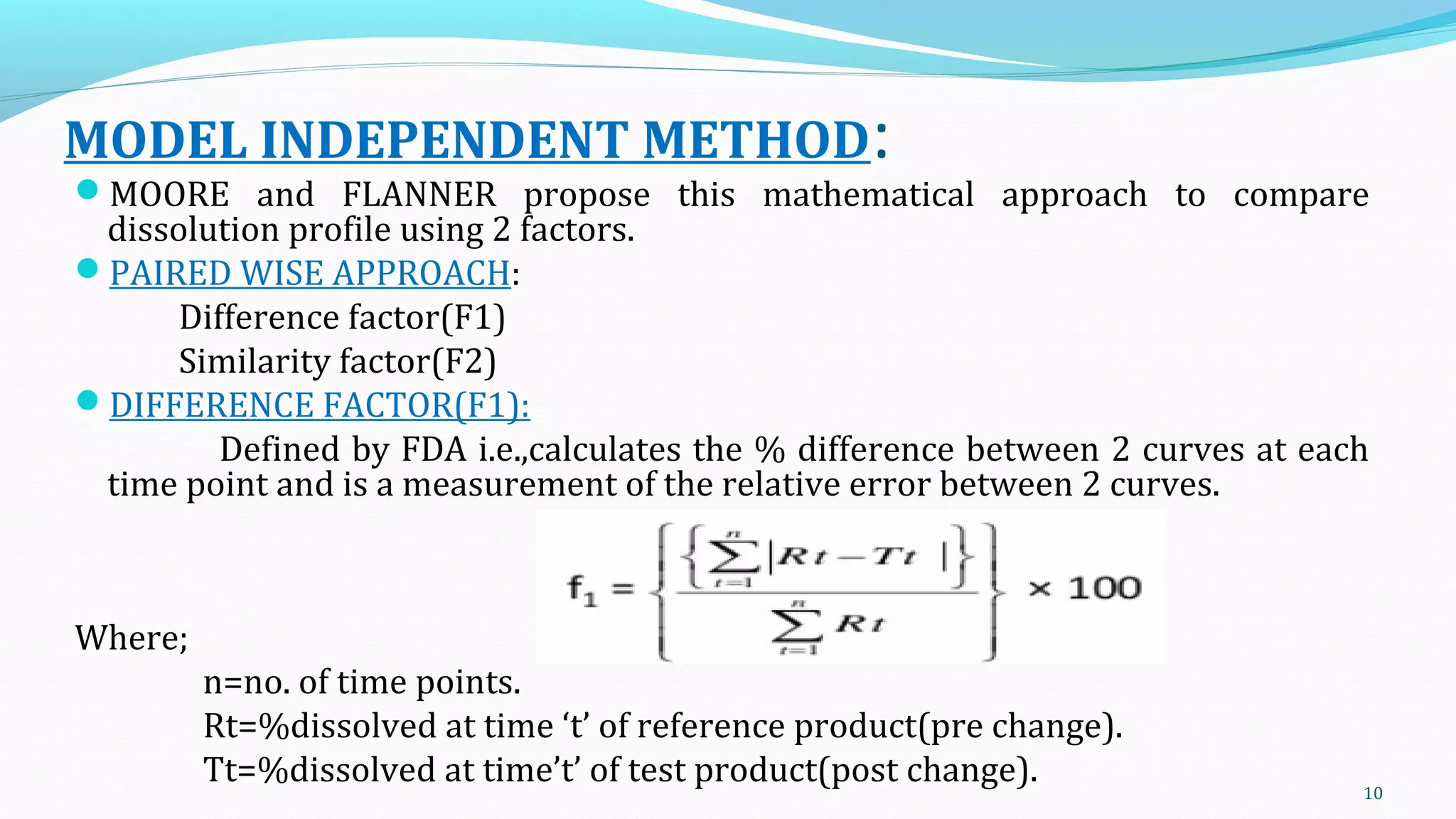
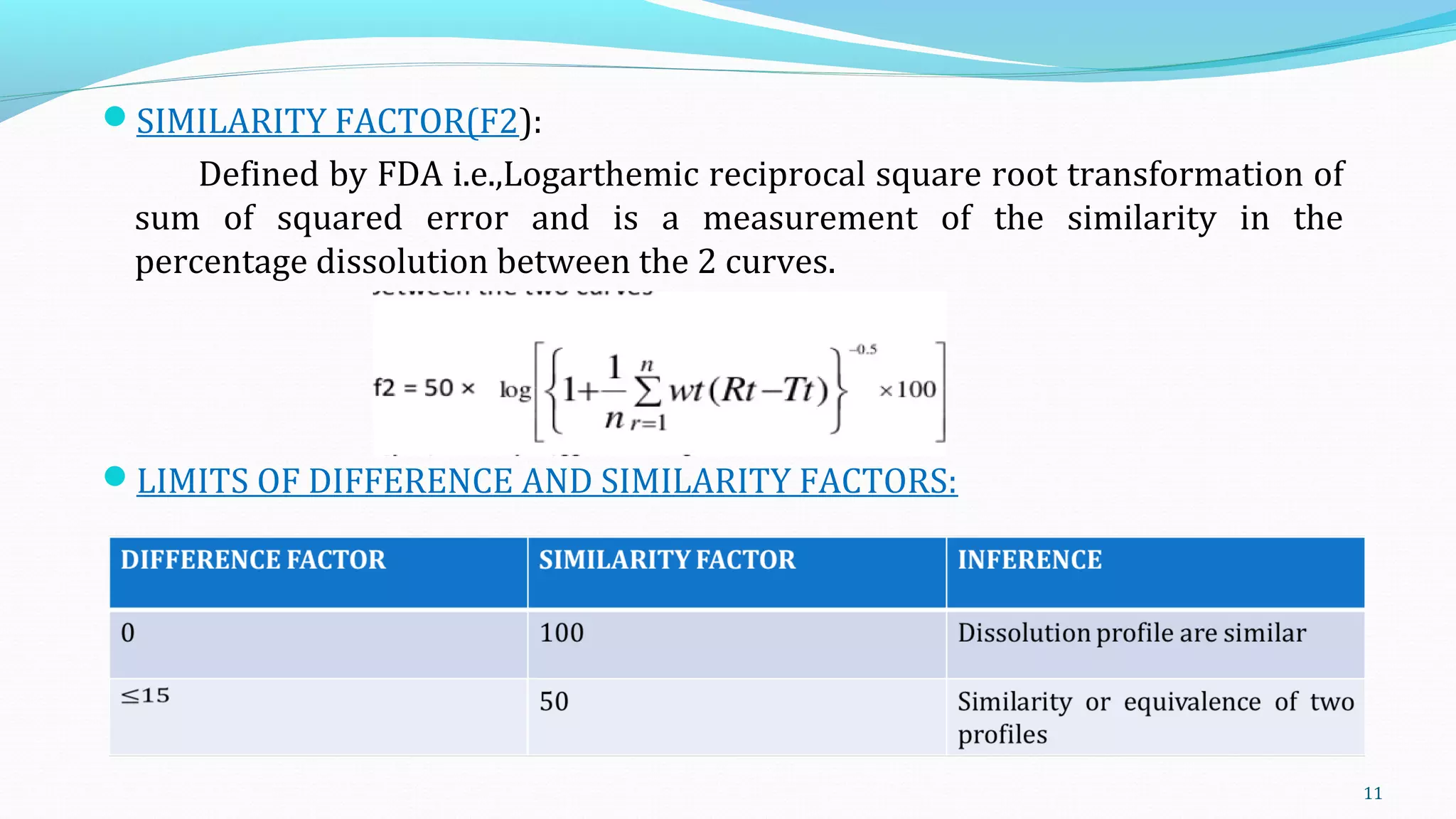
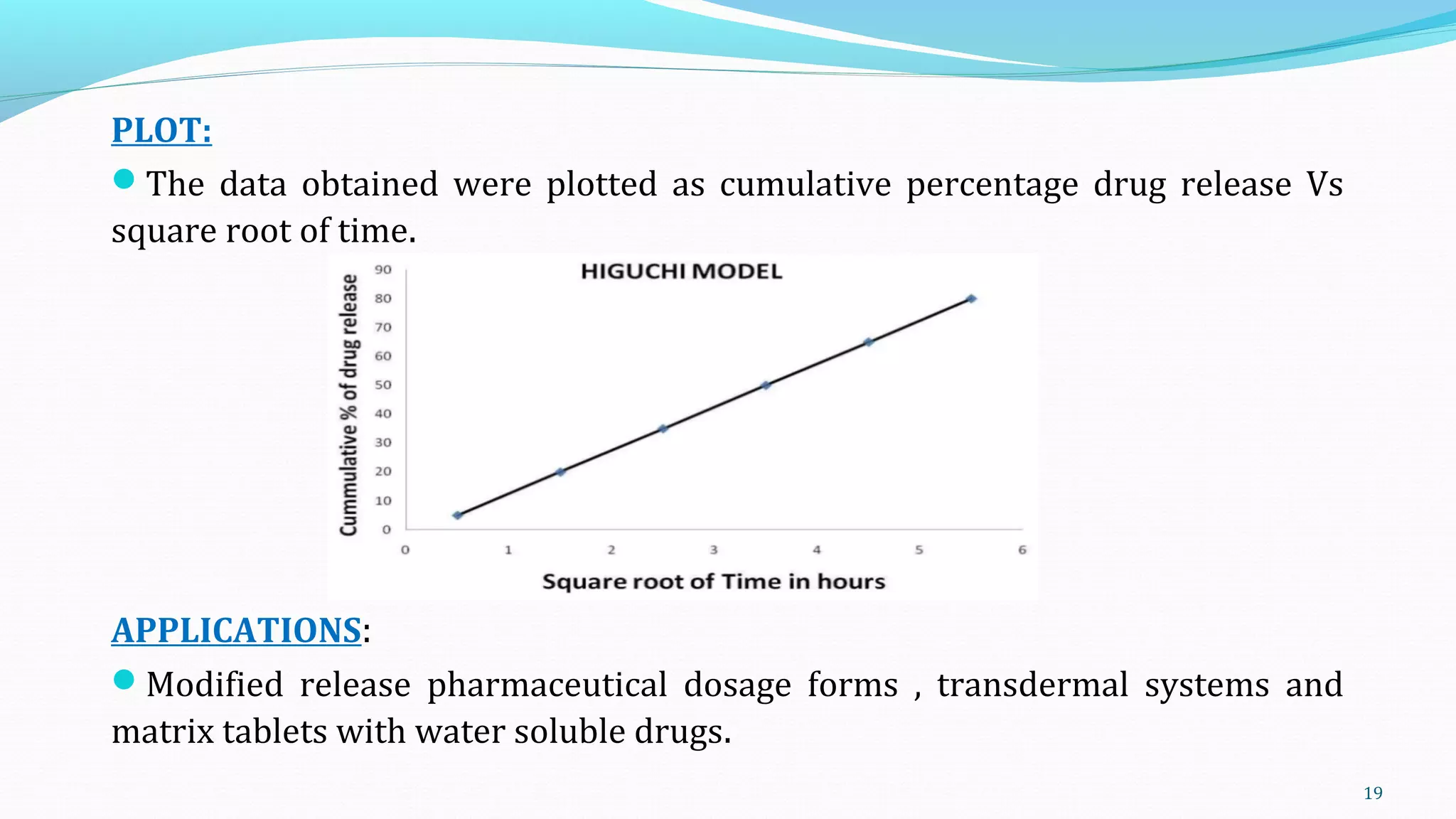
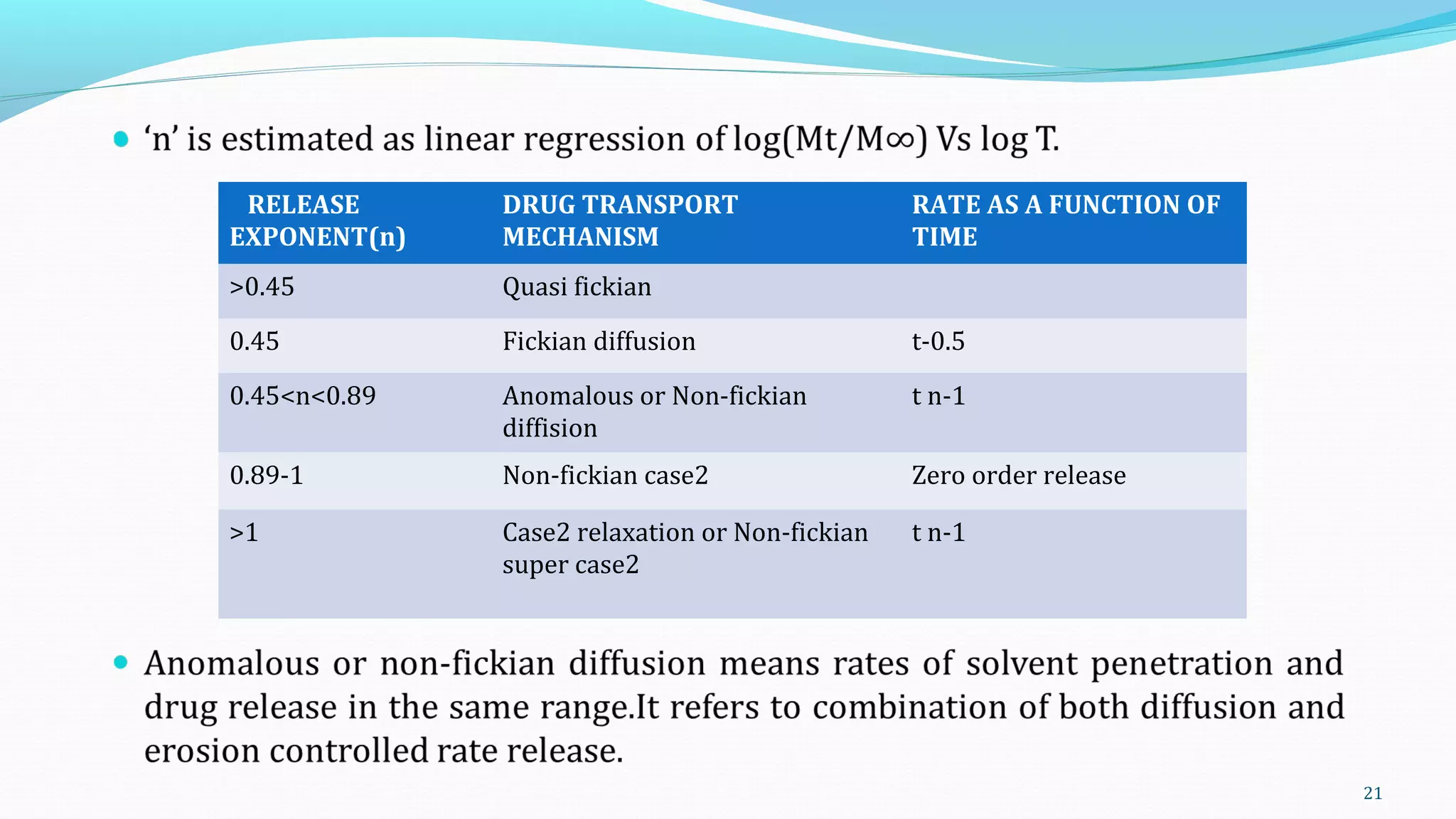
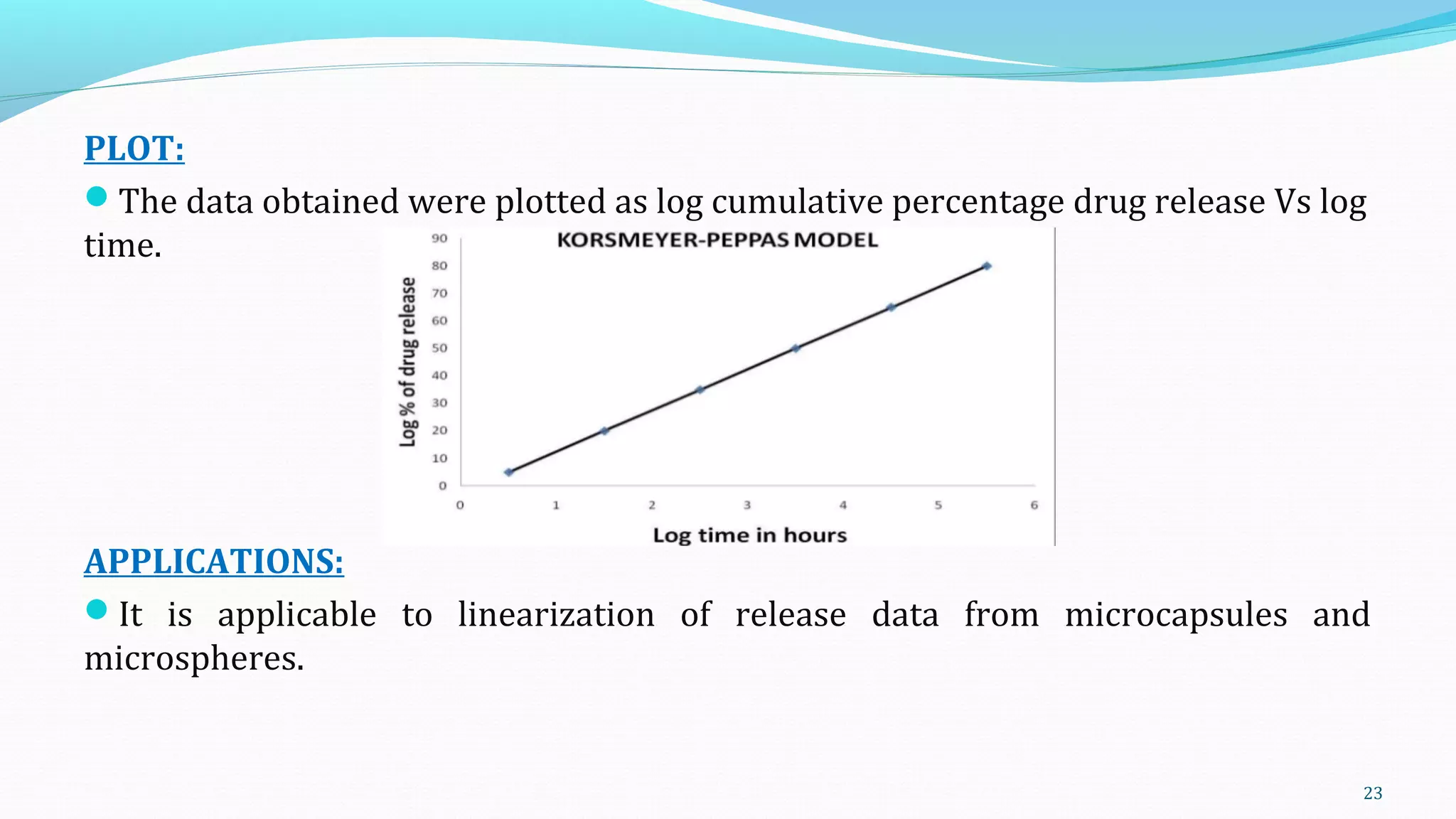
This document discusses various mathematical models used to study consolidation parameters and drug release from pharmaceutical formulations, including the Heckel, Higuchi, Korsmeyer-Peppas, and similarity factor (F1 and F2) models. It provides details on interpreting Heckel plots, limitations of the models, and their applications in understanding drug release mechanisms and comparing dissolution profiles. The summary concludes that these models are important tools for predicting drug release behavior from different drug delivery systems.