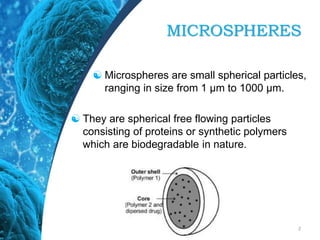
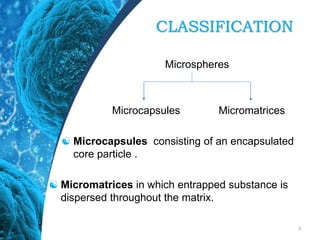
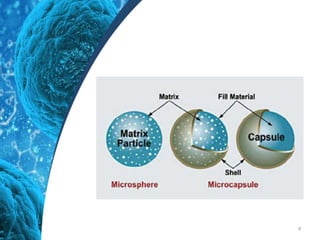
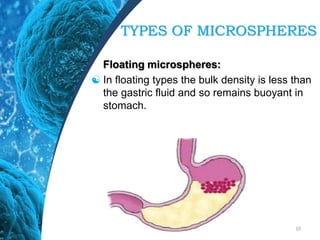
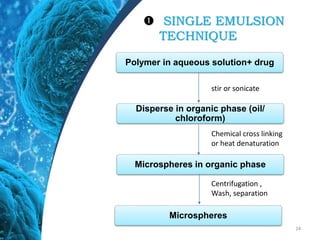
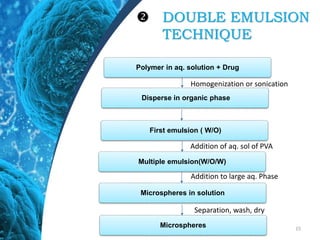
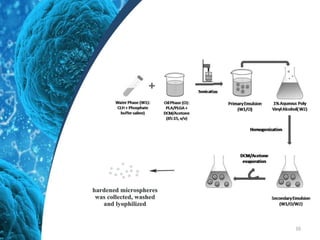
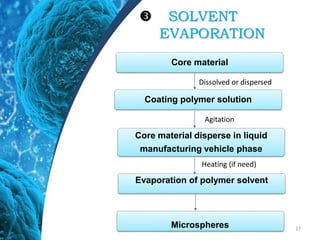
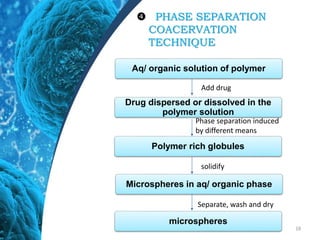
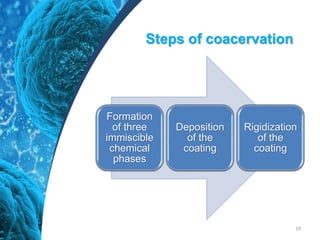
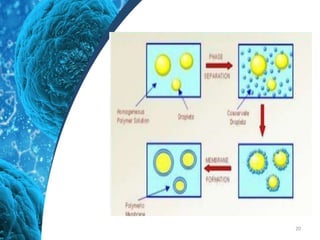
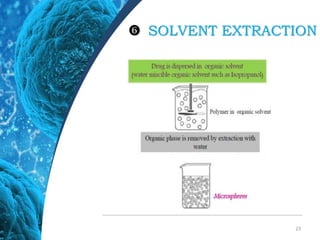
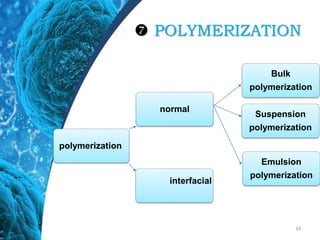
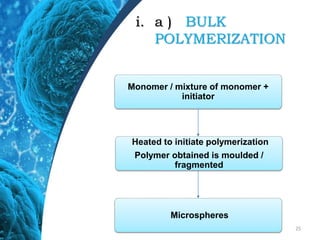
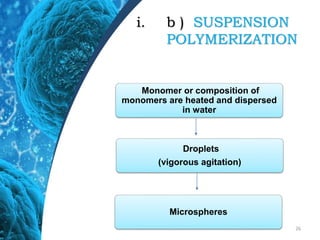
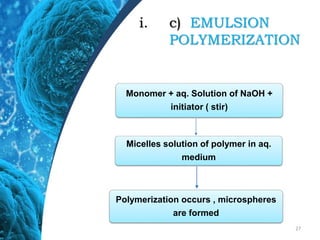
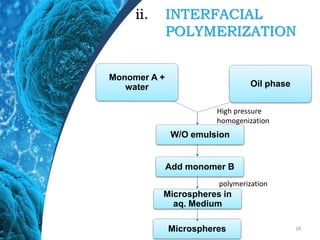
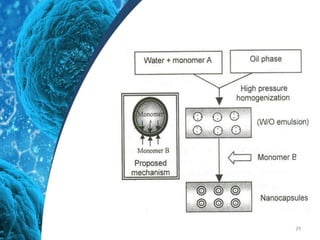
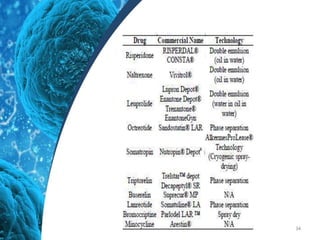
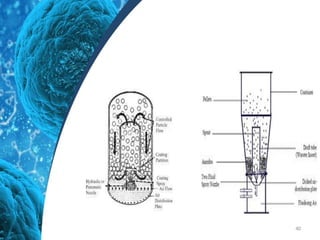
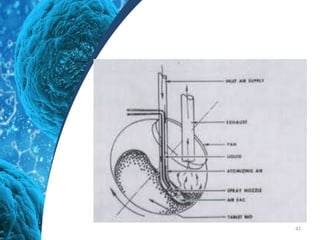
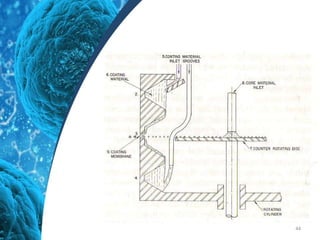
This document provides an in-depth overview of microspheres, which are small spherical particles made from biodegradable materials and used in drug delivery systems. It discusses various types of microspheres, their advantages and disadvantages, classification, preparation methods, and applications including ocular, oral, and transdermal drug delivery. Additionally, it explains the evaluation of microspheres through various analyses to assess particle attributes and drug entrapment efficiency.