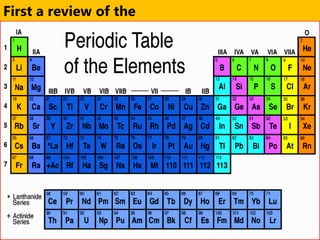
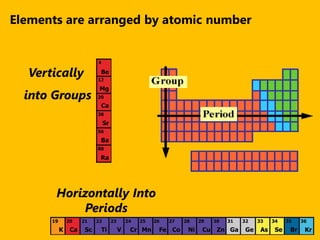
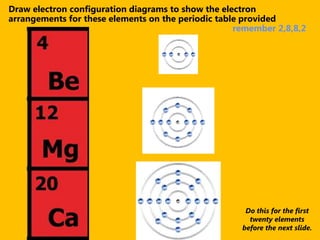
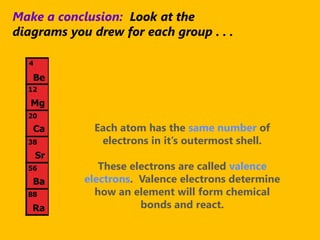
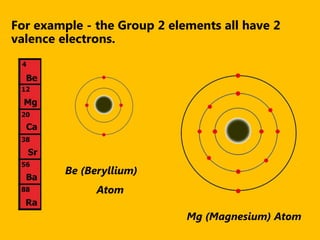
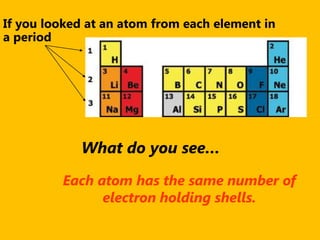
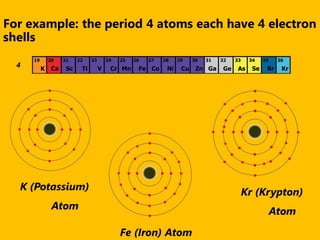
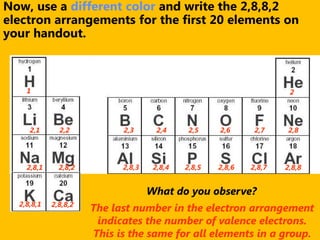
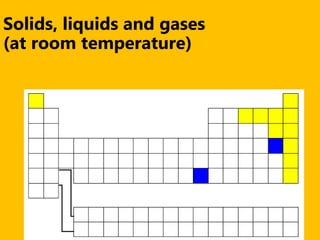
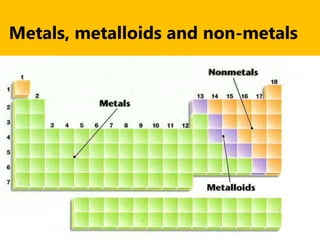
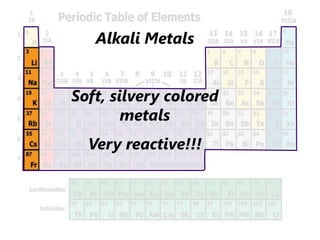
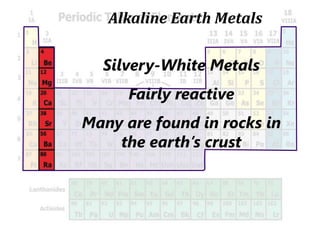
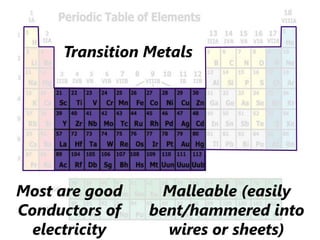
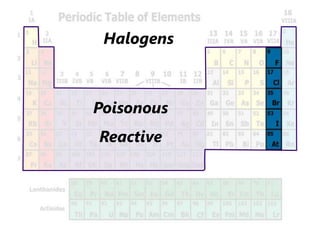
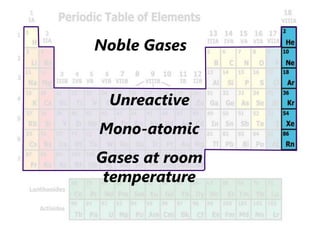
The document introduces the periodic table and discusses how it is organized. It explains that elements are arranged by atomic number vertically into groups and horizontally into periods. [The document] instructs the reader to draw electron configuration diagrams for the first 20 elements and observe that elements in the same group have the same number of valence electrons. It also notes that elements in the same period have the same number of electron shells. In conclusion, [the document] emphasizes that the number and arrangement of valence electrons determines how an element bonds and reacts chemically.