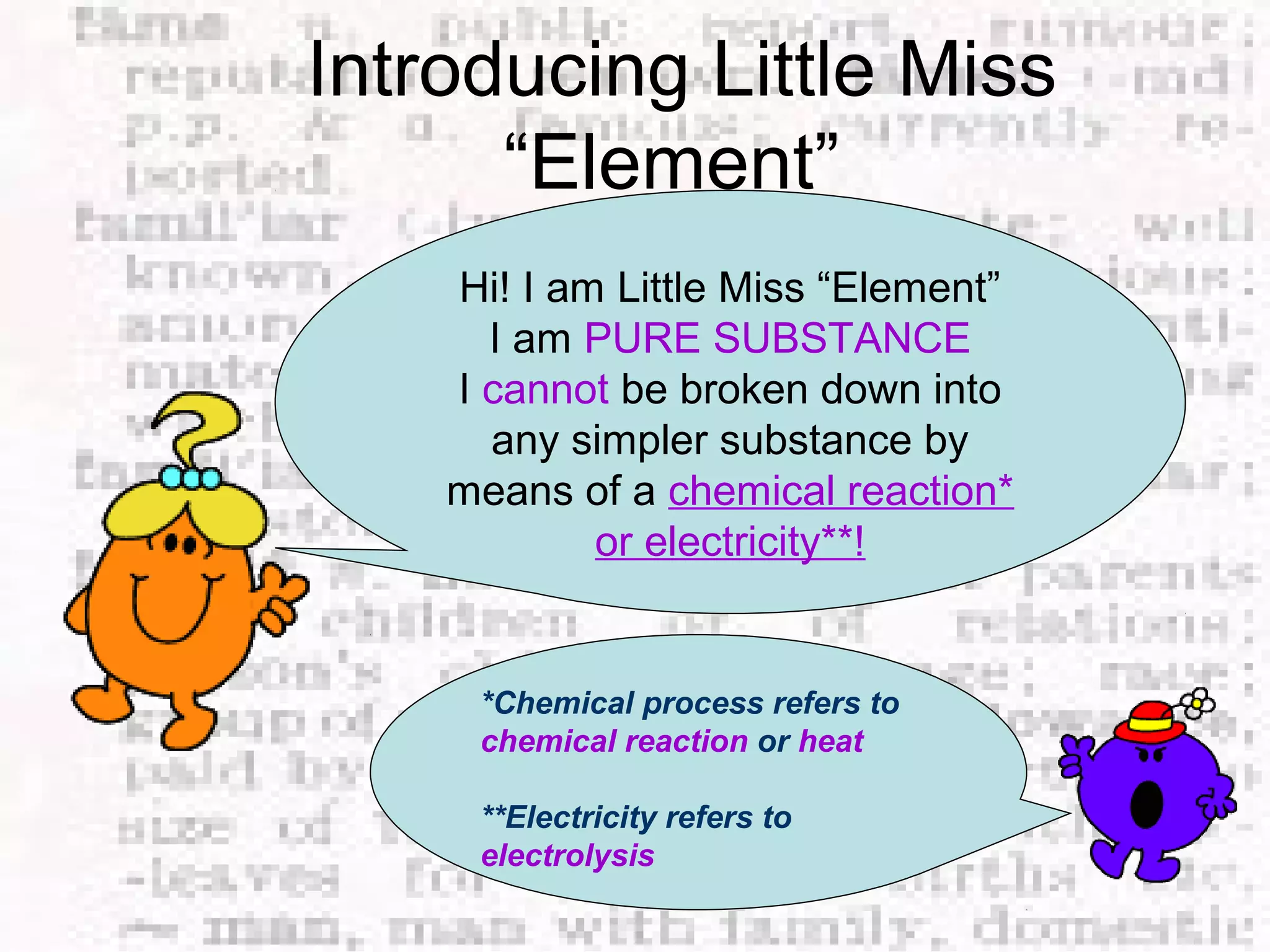
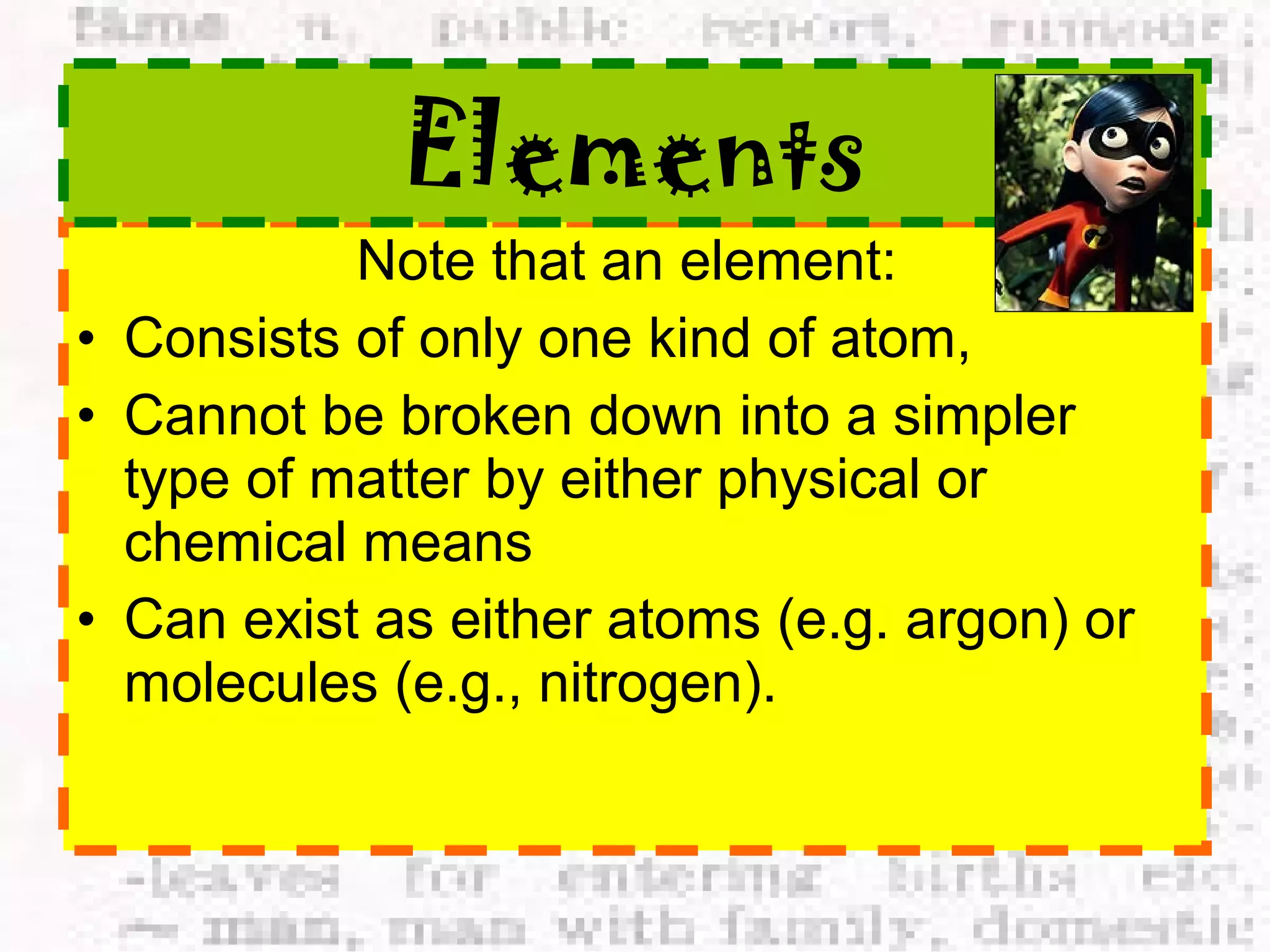
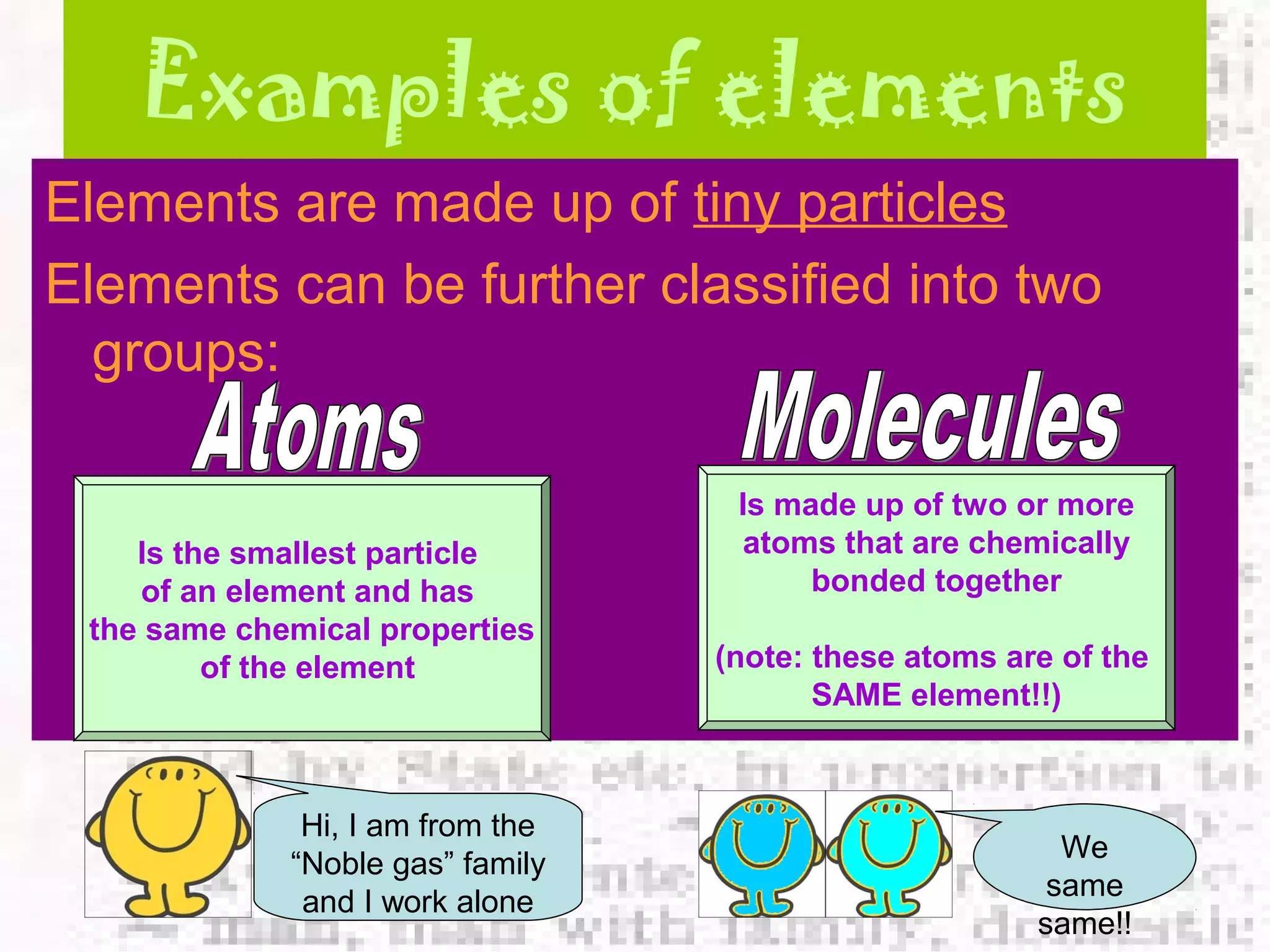
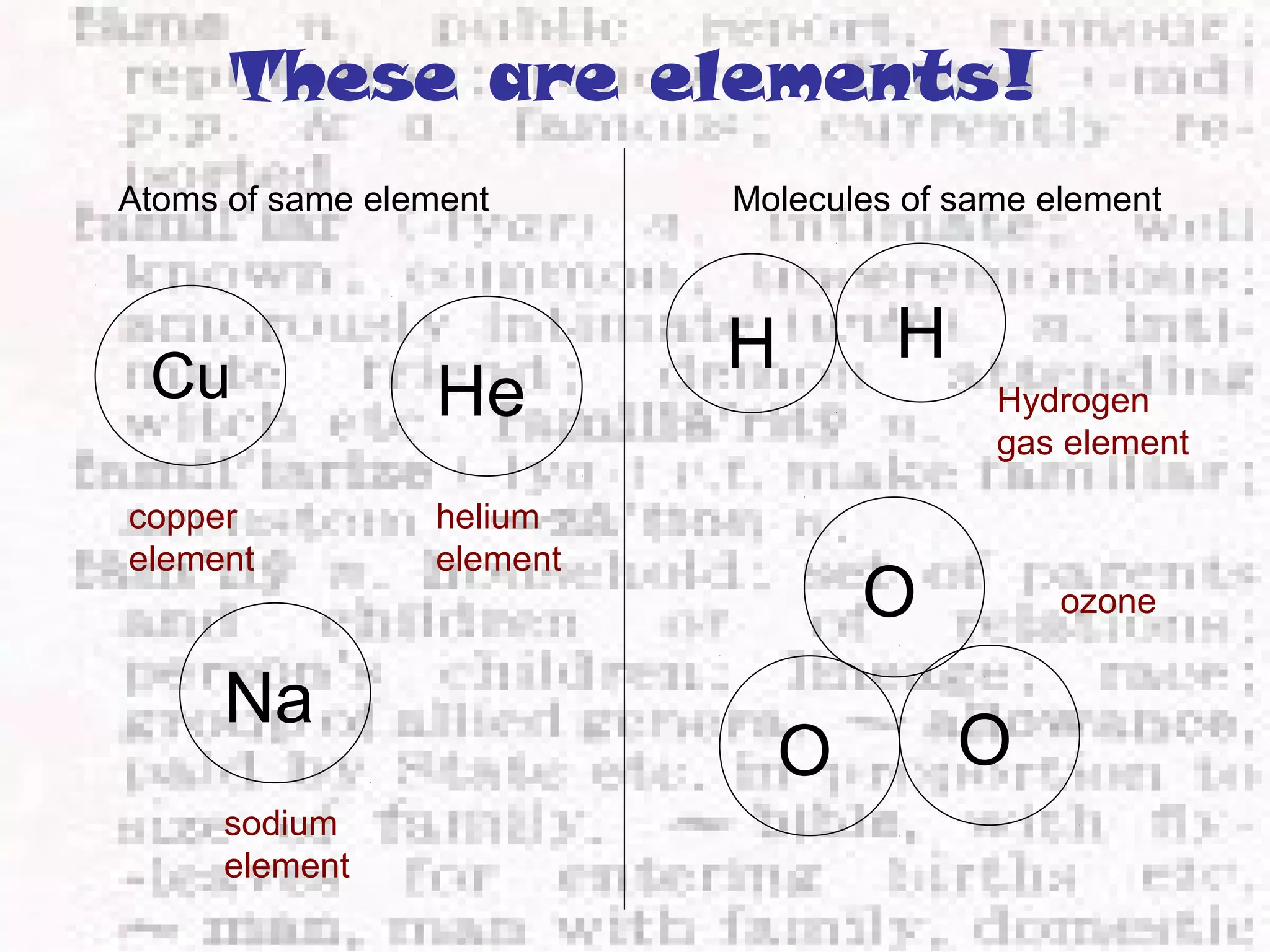
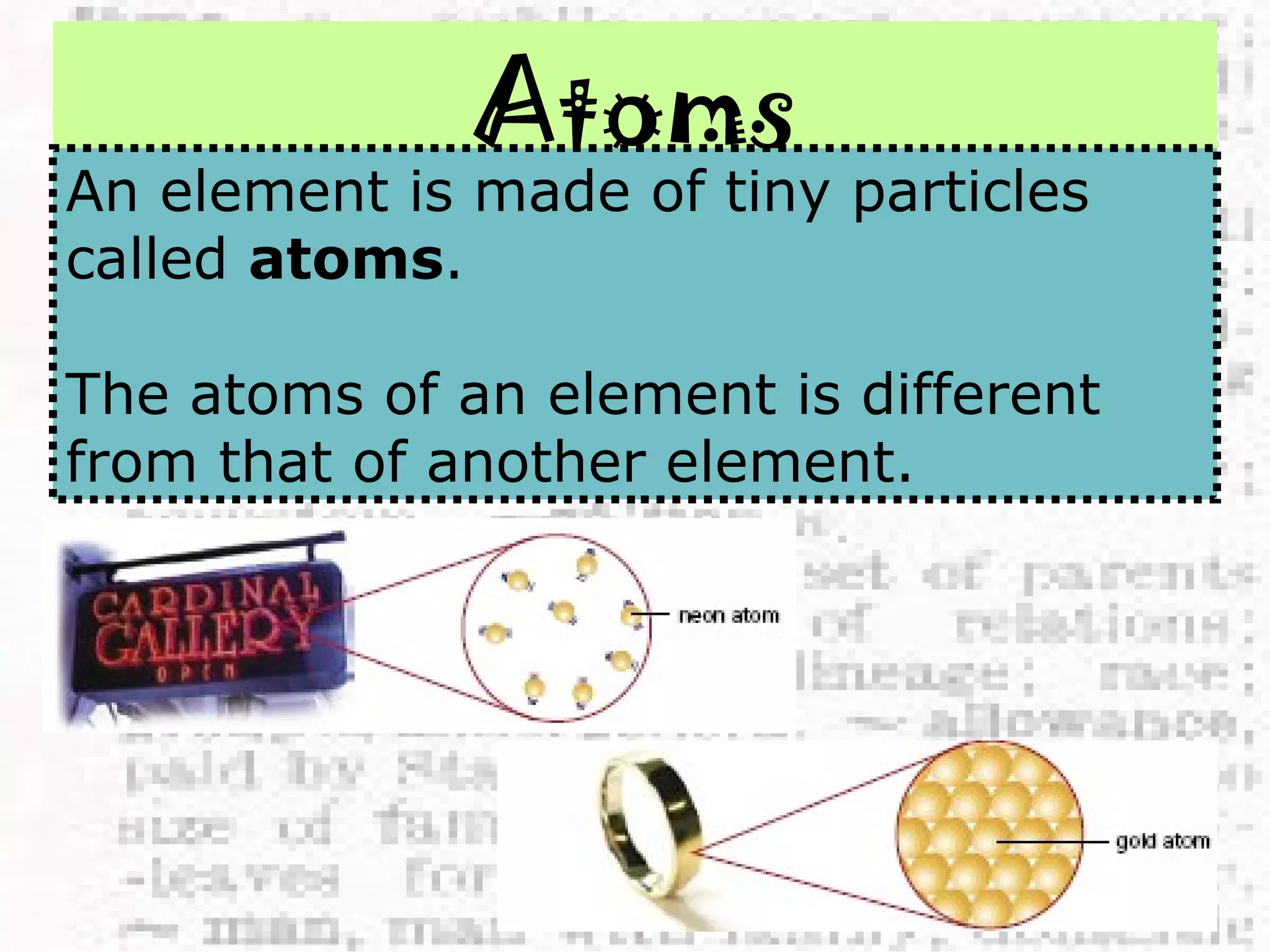
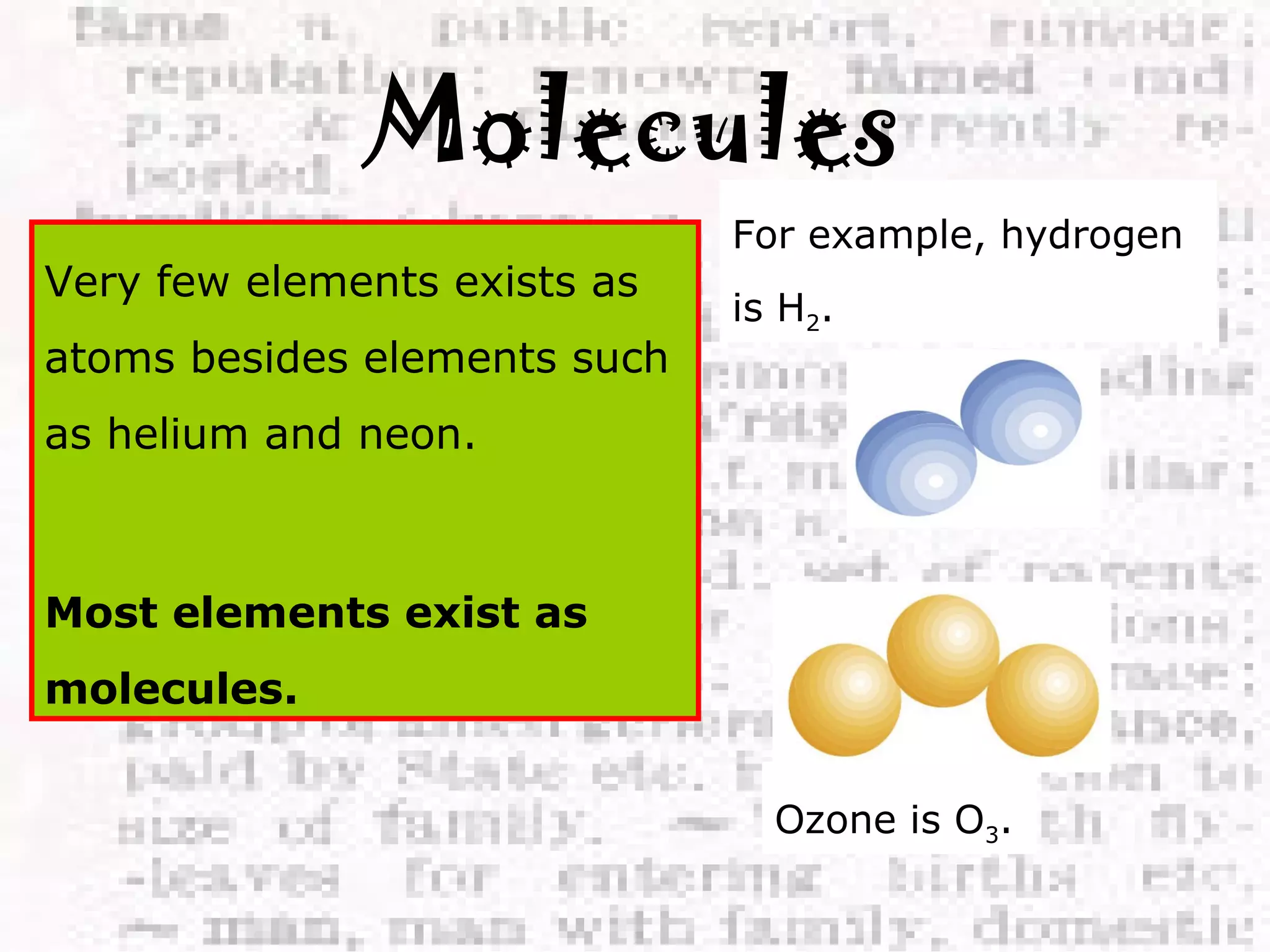
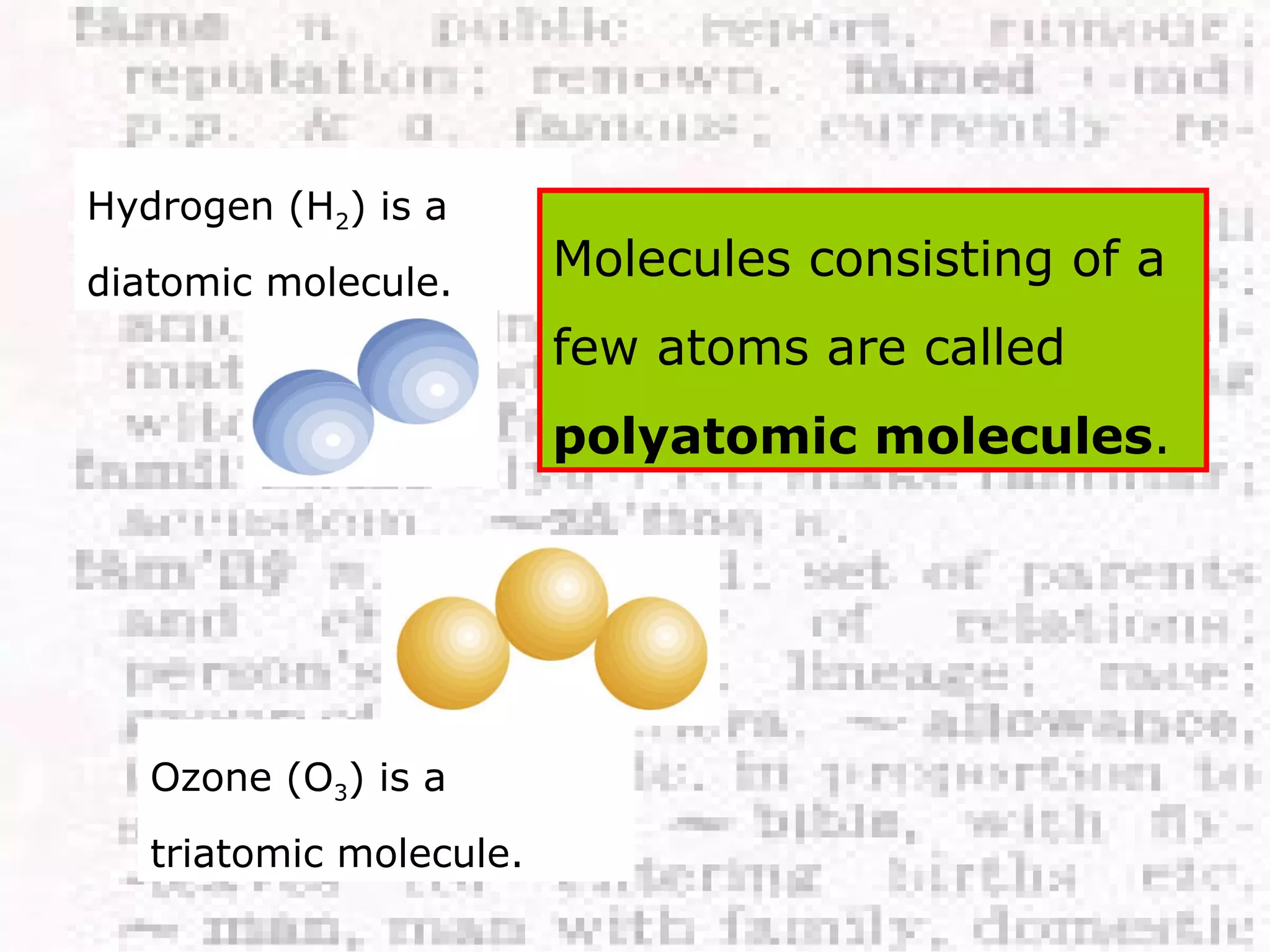
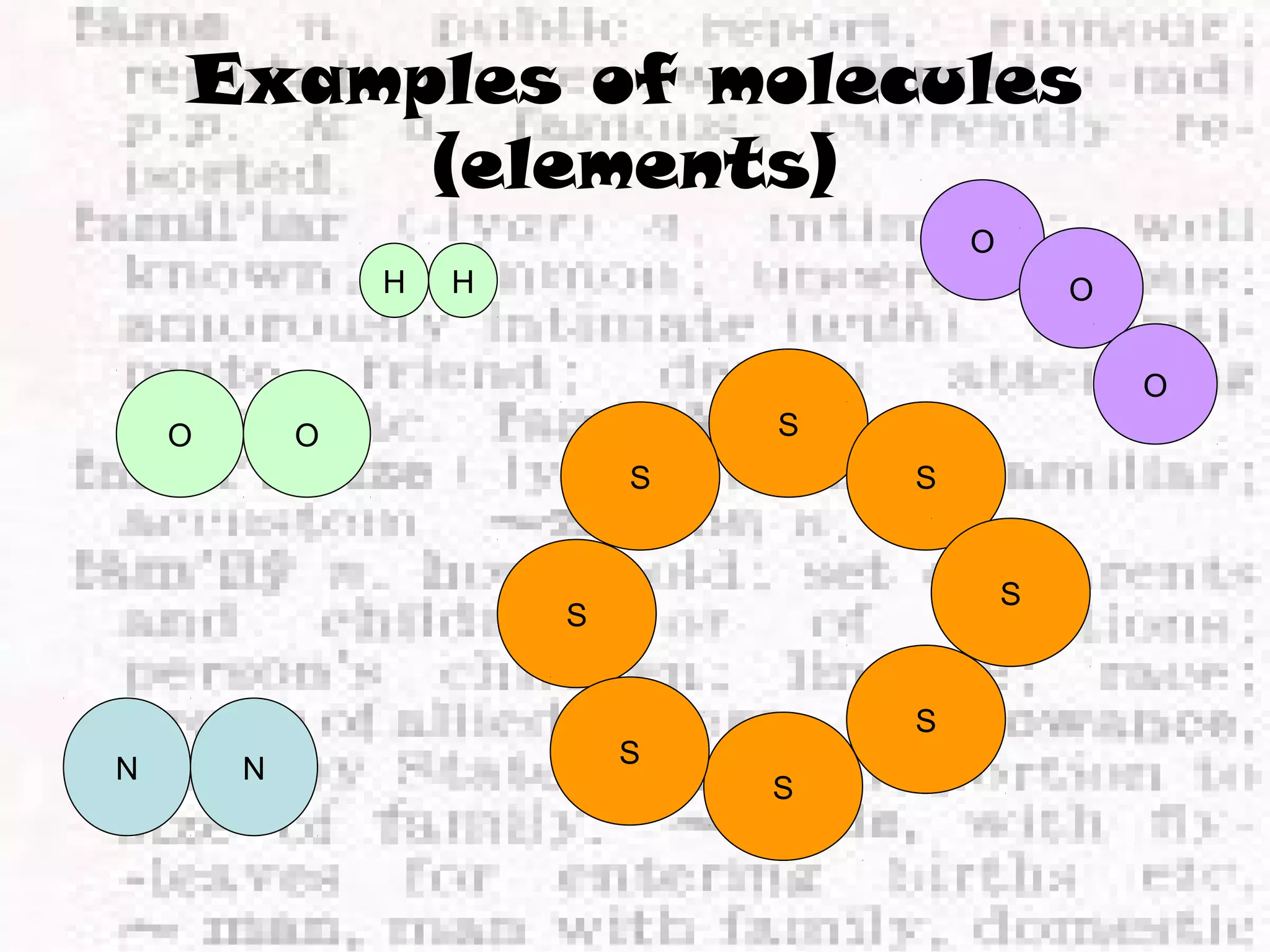
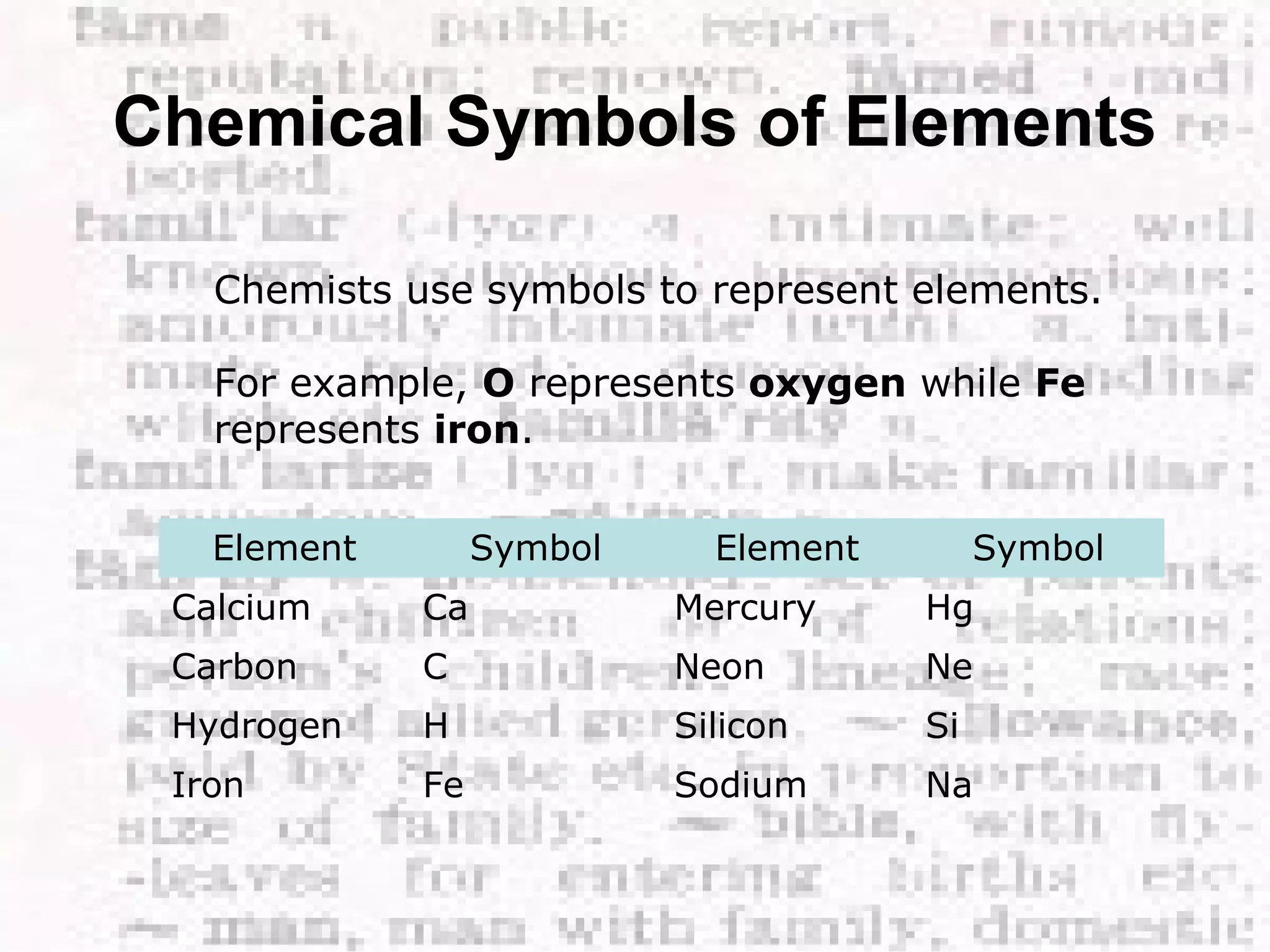
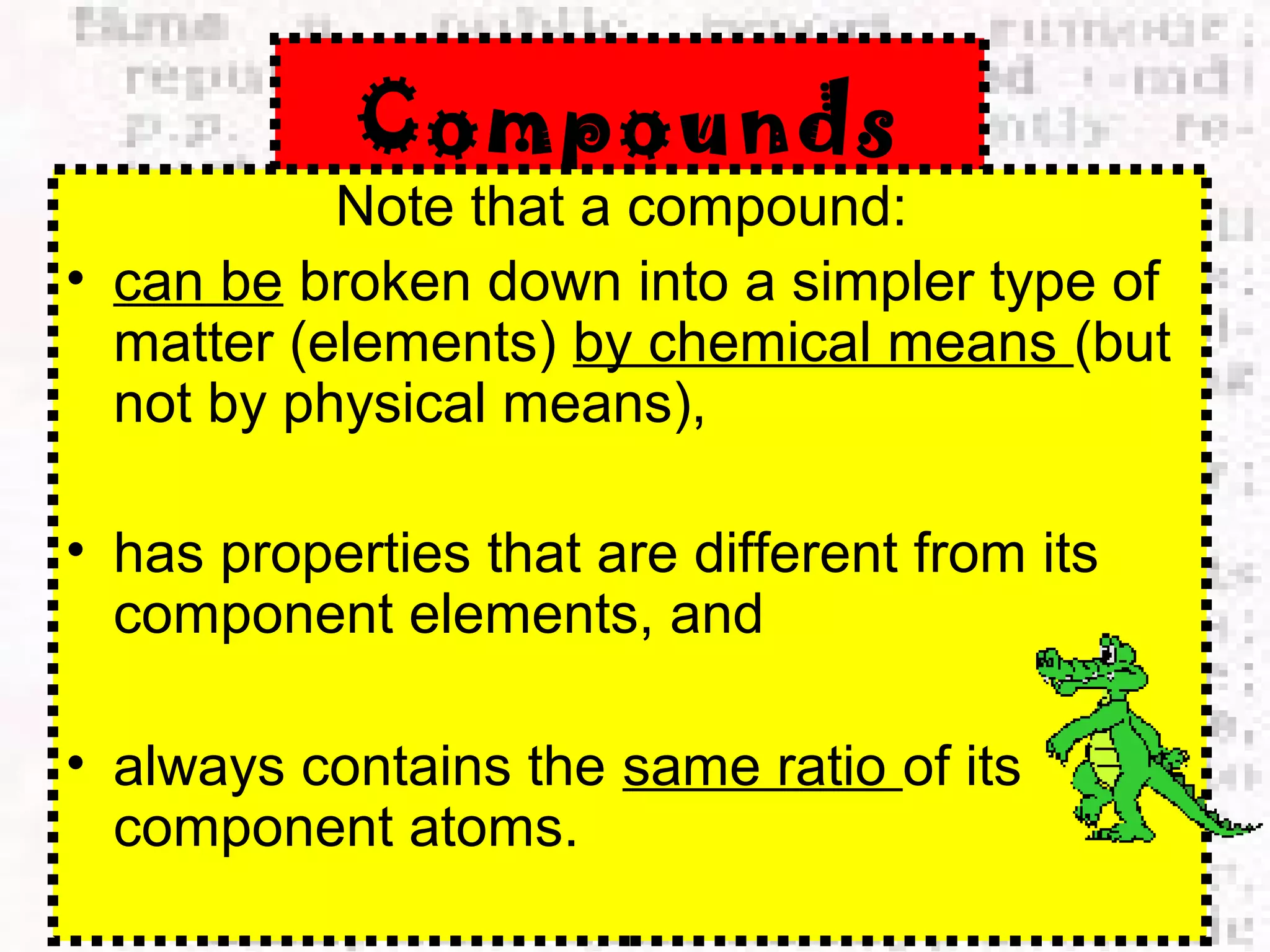
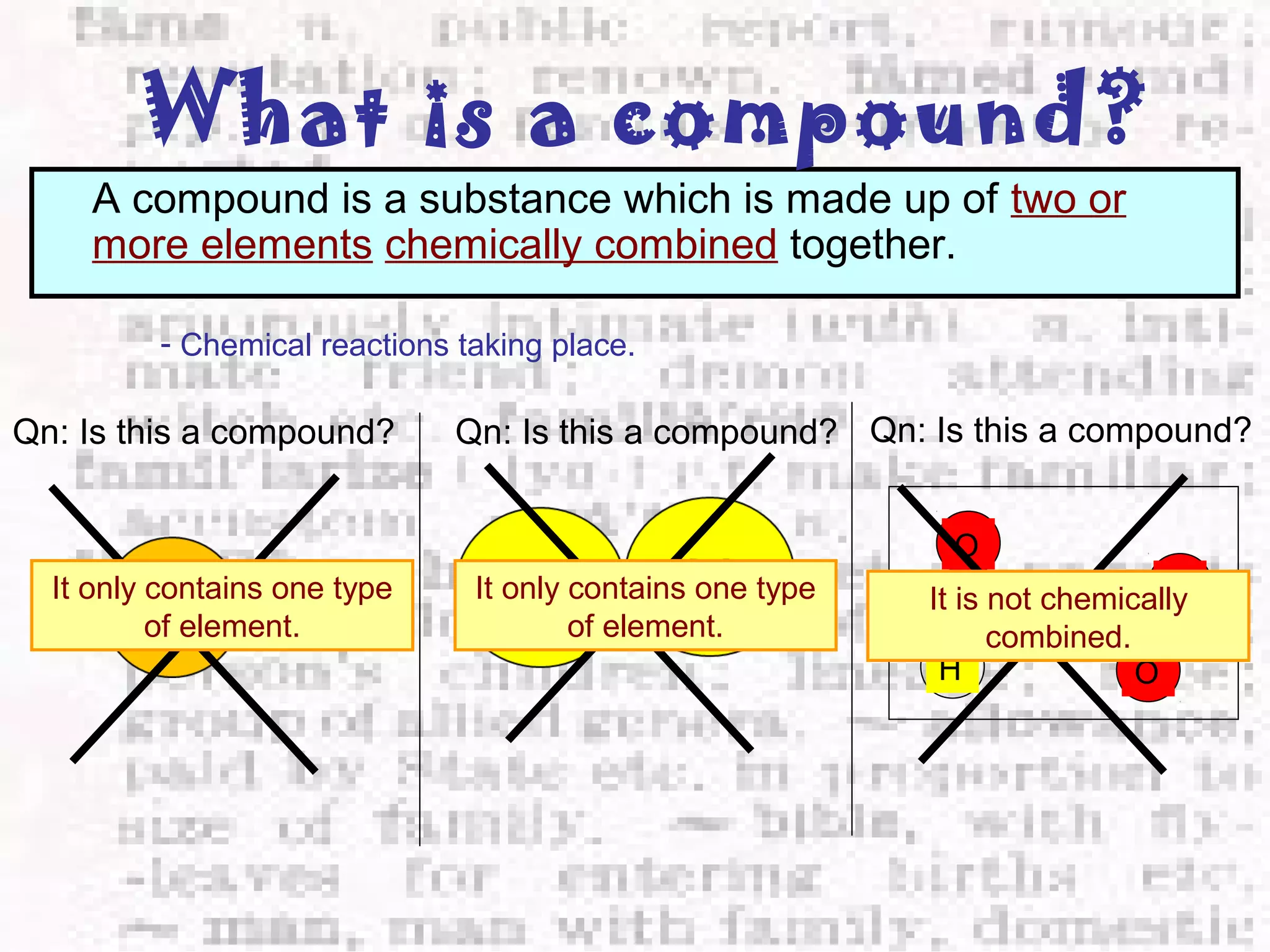
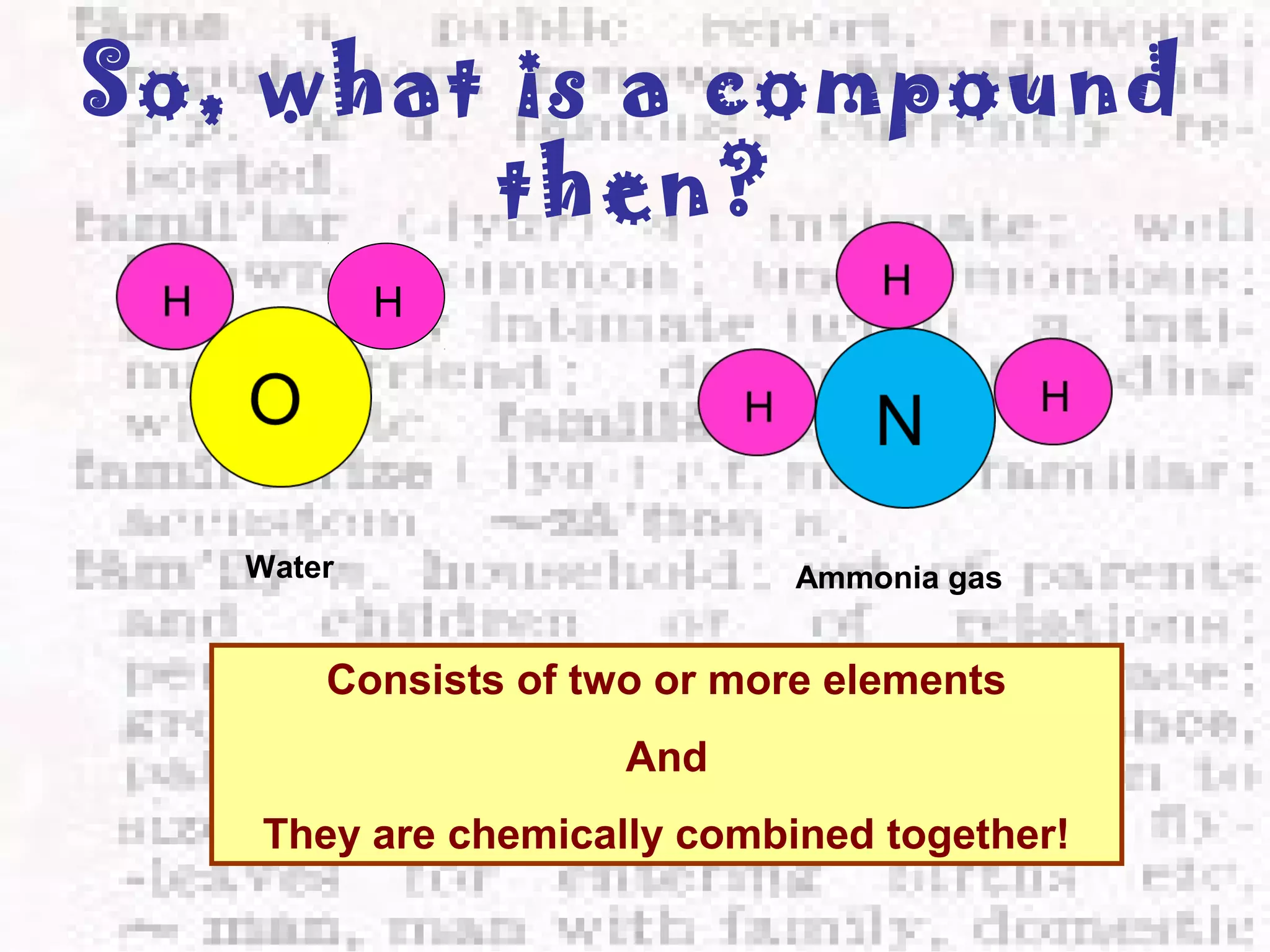
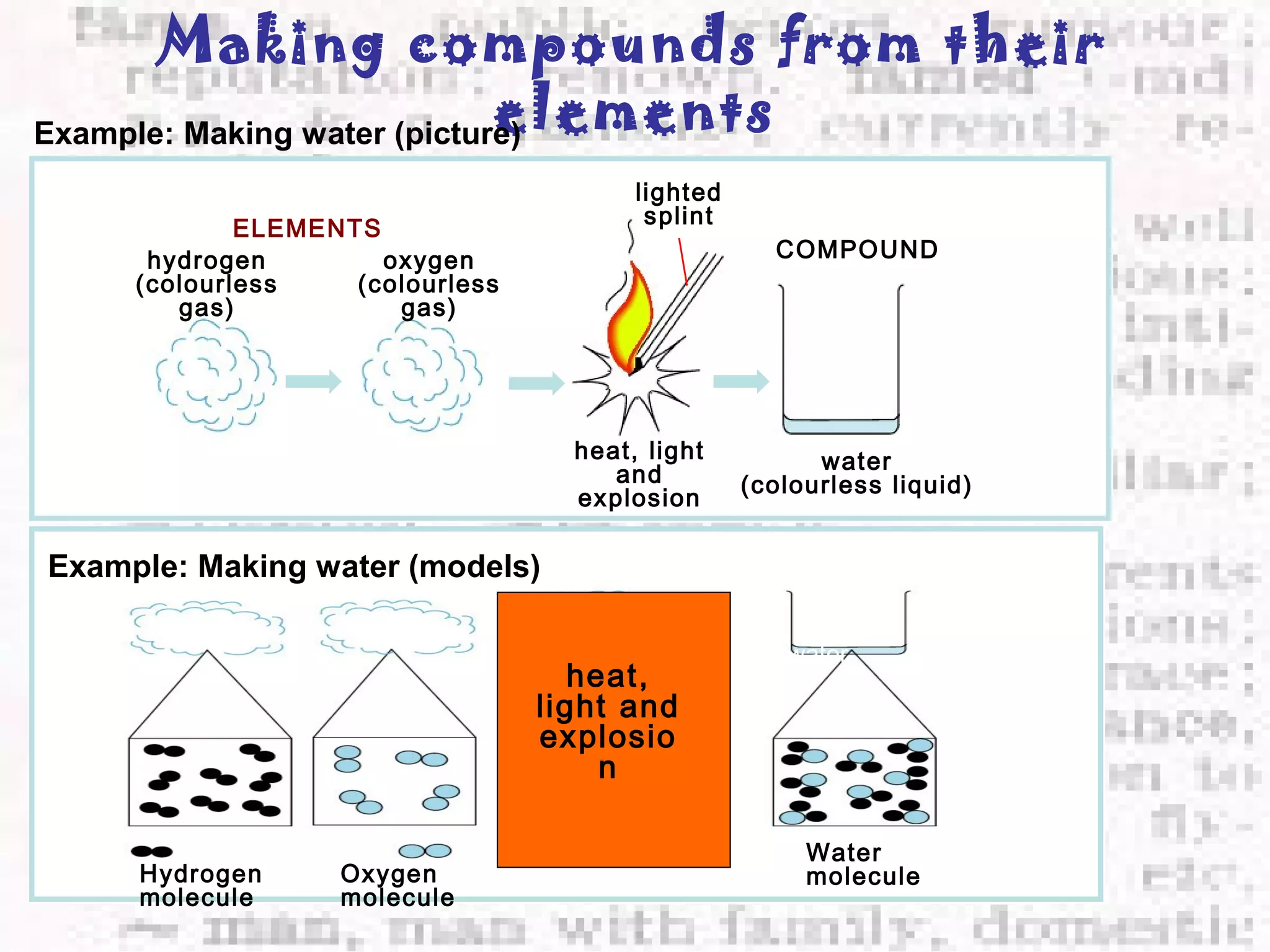
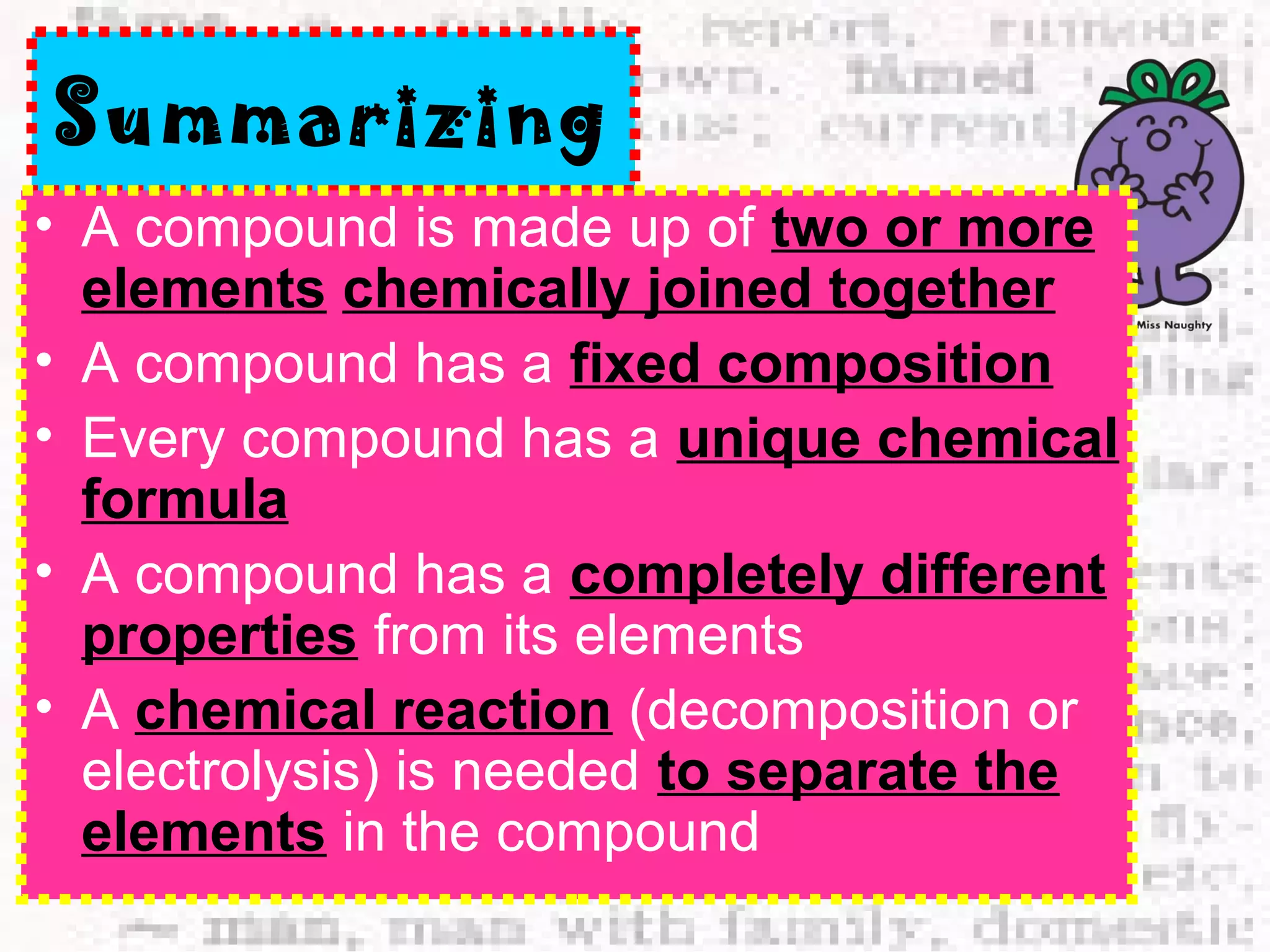
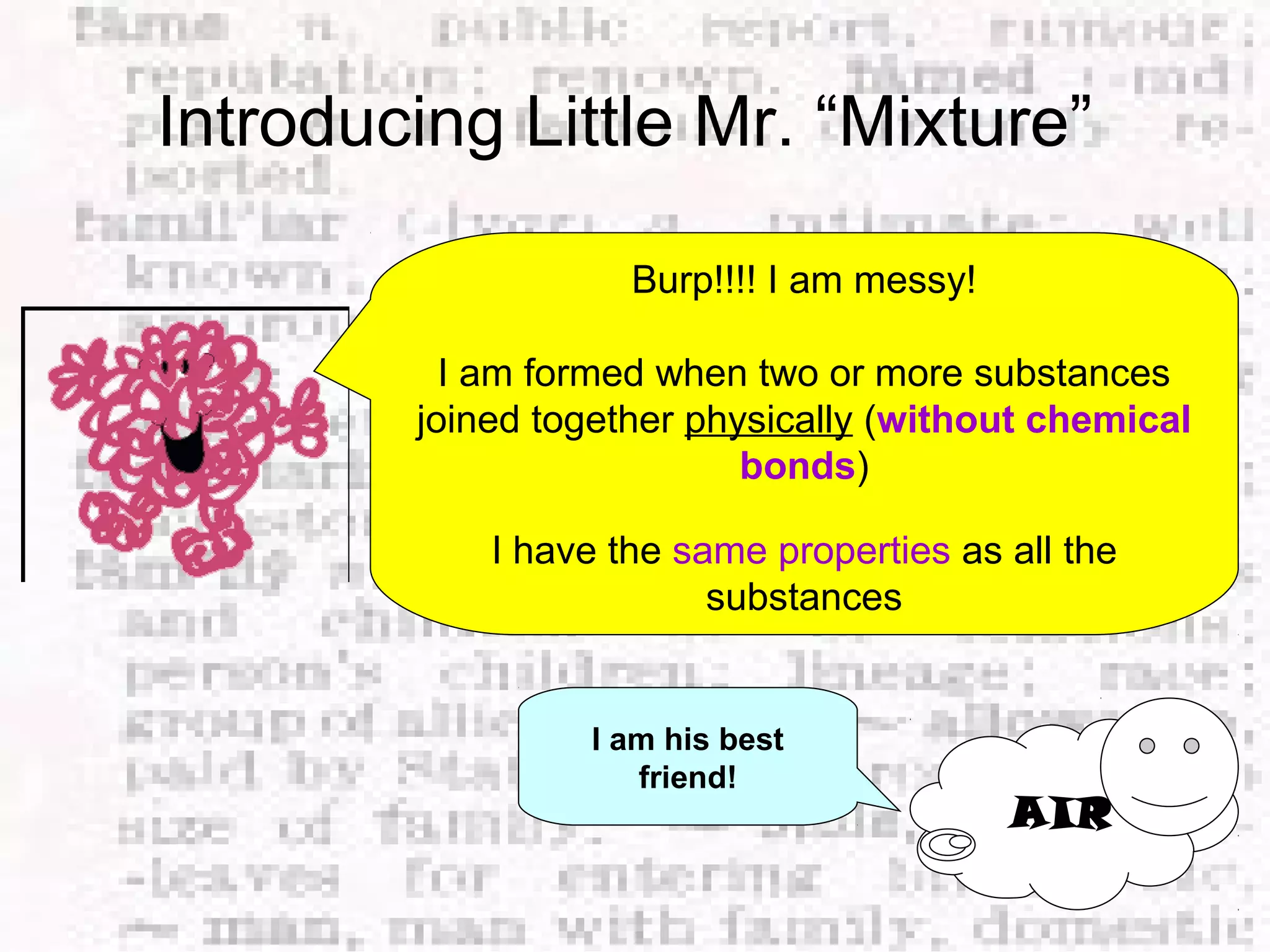
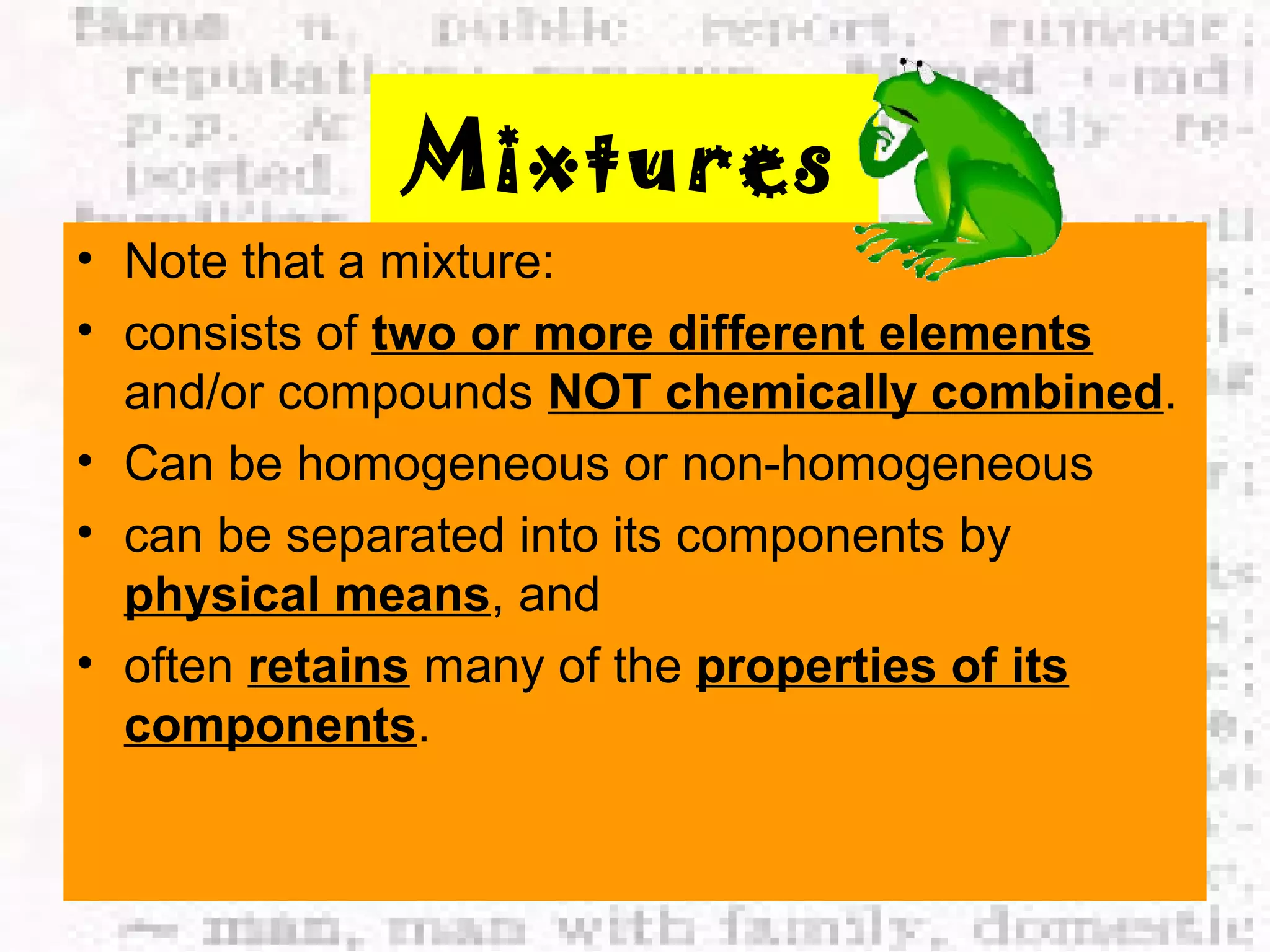
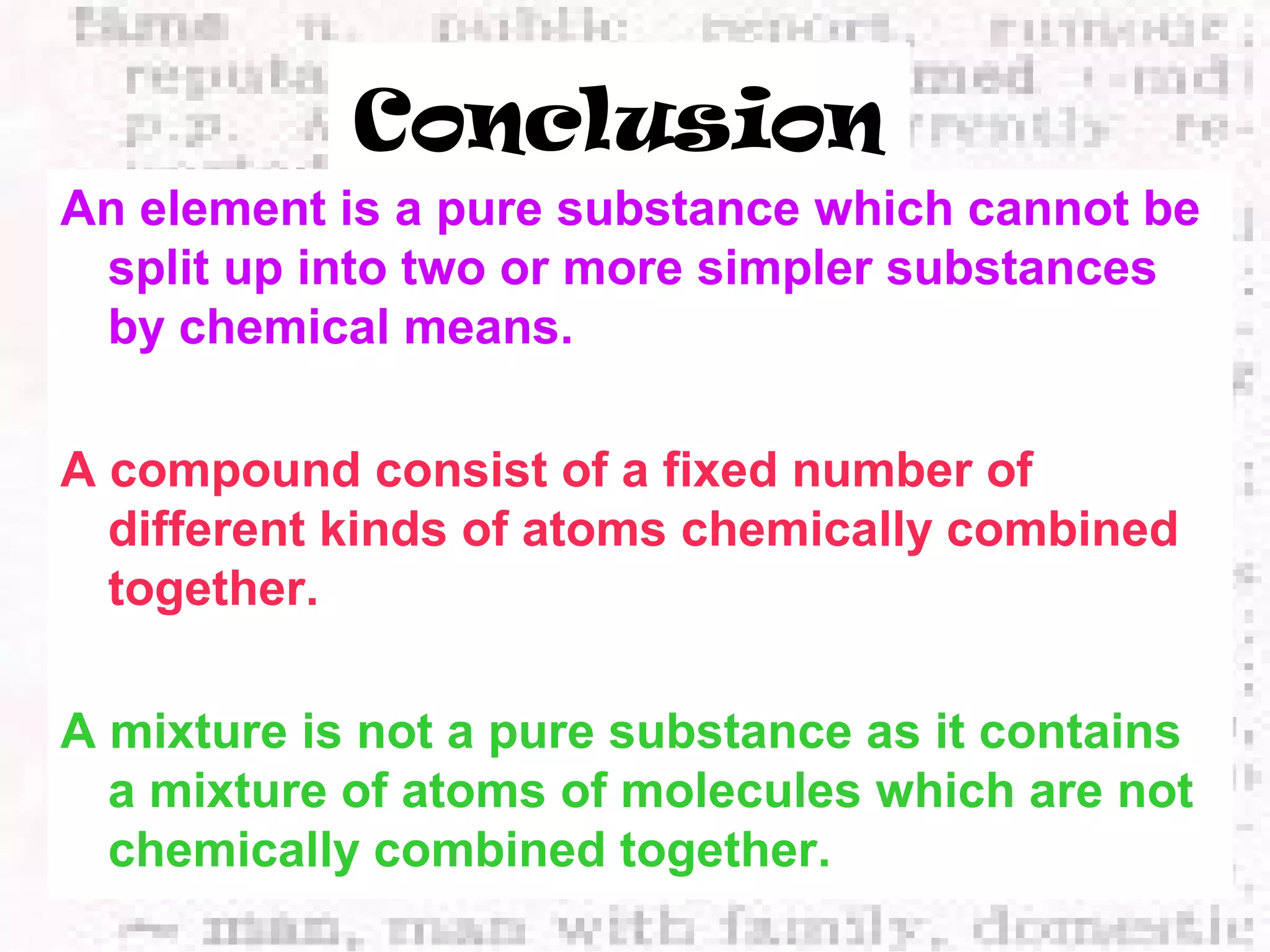
The document defines and provides examples of elements, compounds, and mixtures. Elements are pure substances that cannot be broken down further, such as hydrogen atoms. Compounds are pure substances composed of two or more elements chemically bonded together, like water. Mixtures are physical combinations of elements or compounds that can be separated, such as air or muddy water. The document uses characters like "Little Miss Element" to distinguish the three classifications and provide examples like sodium, iron sulfide, and rojak to illustrate their properties.