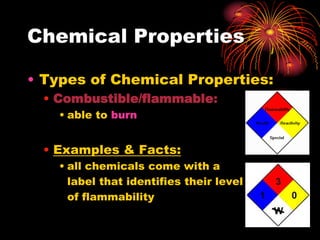
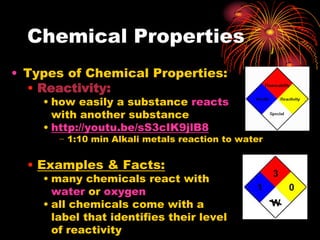
This document defines and provides examples of chemical and physical properties of matter. Chemical properties describe characteristics only observed when a substance changes into another, such as combustibility. Physical properties can be observed without a substance changing, including state, color, odor, and conductivity. The document uses examples like magnesium combustion and metal reactivity to illustrate chemical properties, and discusses physical properties such as luster, brittleness, and solubility. Videos are also included to visually demonstrate different properties.